Abstract
Taxonomy of Pseudohydnum gelatinosum and its sister taxa is revised via morphological data and a four-gene dataset (ITS, nc LSU rDNA, TEF1, RPB1). Identity of P. gelatinosum and Tremellodon pusillus is re-established based on newly collected and sequenced material from their type localities. Pseudohydnum alienum from Europe; P. umbrosum from temperate East Asia; P. cystidiatum, P. meridianum, and P. placibile from Vietnam; and P. omnipavum from North America are described as new to science; P. translucens and P. brunneiceps from East Asia are redescribed. Most Pseudohydnum collections from North America belong to P. gelatinosum ssp. pusillum. A significant divergence of TEF1 sequences in P. gelatinosum is discussed.
Similar content being viewed by others
Introduction
Pseudohydnum gelatinosum (Scop.) P. Karst. is a jelly fungus recognizable because of its substantial, pale-coloured, stipitate basidiocarps with a spiny hymenophore. The species was considered as having a worldwide distribution, with records from Europe, North America, and South America, as well as Asia, Australia, and New Zealand (Wojewoda 1981). A highly peculiar habit of the basidiocarps and rather uniform anatomical structure of specimens collected in different continents maintained this viewpoint although Bourdot and Galzin (1927) pointed at morphologically deviating collections of P. gelatinosum from Europe. Holtermann (1898), Hennings (in Warburg 1899), Martin (1944), Lowy (1959, 1971), and Courtecuisse and Lowy (1990) described aberrant specimens of P. gelatinosum from the tropics. Recently, Chen et al. (2020) introduced a new species from the southeastern part of China, P. brunneiceps Y.L. Chen, M.S. Su & L.P. Zhang, albeit identity of P. gelatinosum elsewhere remained unquestioned. Thereafter, Zhou et al. (2022, 2023) introduced eight more Pseudohydnum species from China, Australia, and New Zealand. A high divergence of P. gelatinosum DNA sequences in public repositories induced us to study the identity of this species and related taxa more closely. Because of the lack of recent material from Slovenia where P. gelatinosum was originally described from, we conducted extensive collecting there in 2019–2020 and supplemented it with sampling in Asia (Siberia, Russian Far East, and Vietnam), other parts of Europe, and North America. Here, we present results of this study.
Material and methods
Morphological study
Specimens from herbaria BPI, GB, H, LE, LJF, PC, TAAM, and UPS were studied. Herbarium acronyms are given according to Thiers (2022). Microscopic routine and terminology follow Spirin et al. (2020). For microscopic study, small cross-sections of dried basidiocarps were rehydrated for 30–40 min and then mounted in lactic acid–based Cotton Blue for a few hours. All measurements were made with the use of phase contrast and oil immersion lens (Leitz Diaplan microscope, × 1250 magnification). At least 20 basidia, 20 hyphae from the context, 20 subhymenial hyphae, and 30 basidiospores were measured per each specimen studied. The following abbreviations are used in taxonomic section: L — mean basidiospore length, W — mean basidiospore width, Q′ — L/W ratio, Q — mean L/W ratio, and n — number of measurements per specimens measured.
A matrix with 1440 values was constructed, where each species was represented by at least 30 measurements of the basidiospore length and width. Statistical analysis and scatter plot of these data was performed using the programming language R 3.5.1 (R Core Team 2018) in the software environment RStudio 2022.07.2 (RStudio Team 2022).
DNA study
In total, 54 specimens of Pseudohydnum spp. were selected for molecular sampling (Table 1). The procedure of DNA extraction completely corresponded to the manufacturer’s protocol of the Phytosorb Kit (ZAO Syntol, Russia). The following primers were used for both amplification and sequencing: the primers ITS1F (Gardes & Bruns 1993) and ITS4 (White et al. 1990) for the ITS1-5.8S-ITS2 region, primers EF1-983F and EF1-1567R (Rehner & Buckley 2005) for a part of the TEF1 region, primers RPB1-Af and RPB1-C2f (Matheny et al. 2002) for RNA polymerase II subunit 1 (RPB1), and primers JS1 (Landvik 1996) and LR5 (Vilgalys & Hester 1990) for D1-D3 domains of nc LSU rDNA region. PCR products were purified applying the GeneJET Gel Extraction and DNA Cleanup Micro Kit (Thermo Scientific, USA). Sequencing was performed with an ABI model 3500 Genetic Analyser (Applied Biosystems, USA). Raw data were edited and assembled in MEGA X (Kumar et al. 2018). Molecular studies were carried out at the centre for collective use of scientific equipment “Cellular and molecular technology of studying plants and fungi” (BIN RAS) and Finnish Museum of Natural History, University of Helsinki (Finland).
Phylogenetic analyses
For this study, 53 ITS, 42 nc LSU rDNA, 19 RPB1, and 26 TEF1 sequences were generated (Table 1). Additionally, 72 ITS and 35 nc LSU rDNA sequences, including the outgroup for the order-level phylogeny, were retrieved from GenBank and UNITE (www.ncbi.nlm.nih.gov/genbank/; https://unite.ut.ee/). The sequences were aligned with webPRANK (Löytynoja & Goldman 2010). Highly divergent sites with questionable homology were removed; the final alignments were adjusted manually.
We compiled six datasets for phylogenetic analyses:
-
(1)
ITS + nc LSU rDNA dataset for main lineages in the Auriculariales. The final alignment contained 966 characters of which 211 bp were parsimony informative. Substitution models: SYM + I + G (ITS) and GTR + I + G (LSU),
-
(2)
ITS + nc LSU rDNA dataset for Pseudohydnum spp. The final alignment contained 1254 characters of which 138 bp were parsimony informative. Substitution models: SYM + I + G (ITS) and GTR + I (LSU),
-
(3)
ITS + nc LSU rDNA + RPB1 dataset for Pseudohydnum spp. The final alignment contained 1600 characters of which 111 were parsimony informative. Substitution models: SYM + G (ITS), GTR + I (LSU), and HKY + 1 (RPB1).
-
(4)
ITS dataset for P. translucens complex. The final alignment contained 514 characters of which 29 were parsimony informative. Subtitution model: SYM + I.
-
(5)
ITS dataset for P. gelatinosum complex. The final alignment contained 522 characters of which 23 were parsimony informative. Substitution model: K80 + I.
-
(6)
TEF1 dataset for Pseudohydnum spp. The final alignment contained 656 characters of which 238 were parsimony informative. Substitution model: GTR + G.
The outgroup choice for the order-level phylogeny (Sebacina incrustans (Pers.) Tul. & C. Tul., Sebacinales) was guided by the current JGI Basidiomycota tree (https://mycocosm.jgi.doe.gov/mycocosm/species-tree/tree;_FJDxL?organism=basidiomycota) where Sebacinales were recovered as a sister group of the Auriculariales. Five other trees were midpoint-rooted.
Phylogenetic reconstructions were performed with maximum likelihood (ML) and Bayesian inference (BI) analyses. Before the analyses, a best-fit substitution model for each marker in each of the alignments was estimated based on the Akaike information criterion (AIC) using ModelTest-NG v0.2.0. A partition homogeneity test (PHT) between different datasets was performed with PAUP 4.0b10* (Swofford 2002). The PHT resulted in a p value of 0.01 for combined ITS + TEF1 dataset, indicating that gene sequences are incongruent and these datasets should be analysed separately.
Maximum likelihood analysis was run on RAxML-NG 1.1.0 (Kozlov et al. 2019) with one thousand bootstrap replicates. Bayesian analyses were performed with the MrBayes 3.2.6 software (Ronquist et al. 2012) by implementing three independent runs each with eight chains and 4 million generations (except 8 million for TEF1 dataset) sampling every 2000 generations, temp = 0.1. In all cases, average standard deviation of split distances reached < 0.01, indicating convergence of the runs. All phylogenetic analyses were conducted in CSC–IT Center for Science (Espoo, Finland) multi-core computing environment.
Results
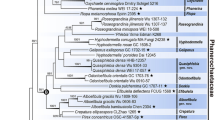
In ITS–nc LSU rDNA-based phylogeny of the Auriculariales, eight described Pseudohydnum spp. and a number of undescribed species recover in the well-supported clade (pp = 1, bs = 83%) (Fig. 1). In ITS–nc LSU rDNA-based phylogeny of Pseudohydnum, the species included in the analysis are divided into seven lineages and sixteen species (Fig. 2). The ITS–nc LSU rDNA-RPB1 phylogeny corroborates this division as far as the two overlap, while providing more information on how these lineages are related (Fig. 3). The lineages (subclades) are as follows (see Fig. 2):
-
(1)
P. totarae (Lloyd) J.A. Cooper known only from New Zealand (Zhou et al. 2022).
-
(2)
P. orbiculare J.A. Cooper and P. tasmanicum Y.C. Dai & G.M. Gates from Tasmania and New Zealand (Zhou et al. 2022).
-
(3)
The third subclade consists of a large cluster of sequences belonging to P. gelatinosum s.l. from the temperate–boreal forests of the northern hemisphere, plus P. abietinum H.M. Zhou & Jung Si and P. sinogelatinosum Y.C. Dai, F. Wu & H.M. Zhou from China. This lineage receives good support in the ITS–LSU–RPB1 dataset (pp = 1, bs = 98%) (Fig. 3), but in the wider ITS–LSU dataset, it is weakly supported in the Bayesian analysis only (pp = 0.92) (Fig. 2). Taxonomic interpretation of this group is difficult. ITS sequences of P. gelatinosum from Eurasia are different in seven positions from the North American ones and up to sixteen positions versus P. abietinum and P. sinogelatinosum. These differences exceed those ones between P. alienum and P. translucens, two sister species from the fifth subclade (see below). As opposed to these two species, European and North American specimens of P. gelatinosum show no differences in the LSU region, and morphology does not allow to separate them either. In the ITS phylogeny of P. gelatinosum complex, European and North American sequences of P. gelatinosum recover in one clade (pp = 1, bs = 85%) (Fig. 4).
Combined phylogenetic ITS–nc LSU rDNA topology from Bayesian analysis showing main lineages within the Auriculariales. All sequences generated for this study are indicated in bold. GenBank accession numbers are given for all additional sequences. Support values (pp/bs) are given above the branches. Scale bar shows expected changes per site
Combined phylogenetic ITS–nc LSU rDNA topology from Bayesian analysis for Pseudohydnum spp. All sequences generated for this study are indicated in bold. GenBank accession numbers are given for all additional sequences. Support values (pp/bs) are given on the branches. Scale bar shows expected changes per site
Combined phylogenetic ITS–nc LSU rDNA–RPB1 topology from Bayesian analysis for Pseudohydnum spp. All sequences generated for this study are indicated in bold. GenBank accession number is given for additional sequence. Support values (pp/bs) are given on the branches. Scale bar shows expected changes per site
-
(4)
Two Vietnamese newly introduced species, P. cystidiatum and P. placibile, comprise this subclade. Both have rather small basidiocarps with a papillate (not hairy) upper surface.
-
(5)
The fifth subclade is represented by the East Asian P. translucens Lloyd (= P. candidissimum H.M. Zhou, T. Bau & Jing Si), the recently introduced P. himalayanum Y.C. Dai, F. Wu & H.M. Zhou and P. sinobisporum T. Bau, H.M. Zhou & Jing Si, and two newly described species from temperate–boreal coniferous forests of the northern hemisphere, i.e., P. alienum from Europe and P. omnipavum from the northwestern part of North America. The latter two species and P. translucens possess pale-coloured basidiocarps, narrow hyphae (if compared with the macroscopically similar P. gelatinosum), and slender hyphidia.
Both ITS–LSU and ITS–LSU–RPB1 phylogenies (Figs. 2 and 6) support separation of closely related P. alienum and P. translucens, but this is not the case with the ITS, as shown by the phylogeny constructed for the P. translucens subclade (data not shown). Reason behind this is that ITS differs only a little between P. alienum and P. translucens (pairwise distance < 1%, 3 bp), while the differences are more robust in LSU (pairwise distance 0.7–1%, 5 bp), RPB1 (pairwise distance < 1%, 5 bp), and TEF1 (pairwise distance 4.8–5%, 21 bp). Morphological traits separating the two species are discussed in taxonomic section (see under P. translucens).
The ITS dataset of P. translucens complex indicates that there seems to be yet more species in this complex. Two highly similar sequences (GenBank KC152166, KT875091) originated from Mexico; however, it is impossible to decide from ITS region only if they belong to one or two species. Four sequences from the northwestern part of North America (UDB034836, UDB0778273, GenBank MF954690, HM488590) have 4–5 unique positions versus P. alienum and P. translucens and show 1–3 bp difference from each other. As in the case of the Mexican sequences, more genetic markers and proper morphological study are necessary to understand how many species they could represent.
-
(6)
This single-species lineage subclade is represented by the newly described P. umbrosum from temperate East Asia. The three-marker phylogeny places this species as a sister to the fifth subclade around P. translucens (Fig. 3). Morphologically, P. umbrosum is distinguishable from other species due to dwarf-sized, dark-coloured basidiocarps with a rudimentary stipe and long, ellipsoid basidiospores.
-
(7)
The last subclade encompasses P. brunneiceps Y.L. Chen, M.S. Su & L.P. Zhang recently described from the southern part of China and a new species, P. meridianum from Vietnam. They both have substantial, dark-coloured basidiocarps with a pronounced, long stipe differentiating them from other members of the genus.
The TEF1 phylogeny (Fig. 5) makes the whole picture more complicated. TEF1 sequences of P. gelatinosum are divided among three clades, and each of them reveals strong inner variation. All but two TEF1 sequences of P. gelatinosum (all originated from Eurasian specimens) belong to two related clades; however, genetic distance between them is comparable to other sister species within the genus. The larger clade covers Eurasia from the northern part of Slovenia up to Kuril Islands, while the smaller clade embraces an epitype of P. gelatinosum from the western part of Slovenia (Spirin 13369) and another specimen from the southern part of the country, as well as three other collections from very distant areas (i.e., Finland, South Ural, and Central Siberia) (Fig. 5). This certainly points at the lack of a geographic pattern reflecting these two genetic groups. No morphological or ecological data differentiate them either. The single TEF1 sequence from the North American specimen of P. gelatinosum (Miettinen 19671) clearly deviates from the Eurasian ones although it forms a strongly supported lineage with TEF1 sequence of P. gelatinosum from Kunashir, Kuril Islands (LE 313565). ITS sequence of the latter specimen shows no differences versus other P. gelatinosum sequences from Eurasia. Therefore, we are unwilling to interpret the high divergence among TEF1 sequences of P. gelatinosum s. lato as an argument for splitting it into several species. Nevertheless, we redescribe the North American specimens of P. gelatinosum as belonging to ssp. pusillum, in anticipation of better solution in the future. In the combined ITS–LSU–RPB1 phylogeny, P. gelatinosum from Eurasia and ssp. pusillum from North America form one highly supported clade (pp = 1, bs = 98%) (Fig. 3). The taxonomic status of P. abietinum and P. sinogelatinosum as separate species deserves further clarification with the use of RPB1 and TEF1 sequences. To conclude, we can only recognize one species in this lineage, P. gelatinosum, with high certainty, while one to two further species (including P. sinogelatinosum) might also be recognized pending better genetic data.
Bayesian phylogram for the TEF1 dataset, showing phylogenetic relationships of Pseudohydnum spp. All sequences generated for this study are indicated in bold. GenBank accession number is given for additional sequence. Support values (pp/bs) are given on the branches. Scale bar shows expected changes per site
Taxonomy
Pseudohydnum alienum Spirin & V. Malysheva, sp. nov. — Figs. 6, 9, and 10
MycoBank MB846386.
Holotype. Russia. Karachay-Cherkessia: Karachayevsk Dist, Teberda Nat. Res., Khadzhibey, 43.37889N 41.68360E, Picea orientalis, 8.VIII.2009 V. Malysheva (LE 253853*, isotype — H 7200585).
Etymology: alienus (Lat., adj.) – alien, unfamiliar.
Basidiocarps up to 2 cm in widest dimension and 2–3 mm thick, pileate, laterally stipitate, gelatinous. Pileal surface strigose, watery-greyish, sometimes with faint reddish-brownish stains, pale ochraceous or greyish-brownish in dry condition. Pileal edge rather blunt, fertile. Spines sharp-tipped, white to greyish, 0.5–1 mm long, 6–8 per mm. Stipe a few millimeter long, watery-greyish, usually covered by spines up to the very base.
Hyphal structure monomitic, hyphae clamped. Hyphae of pileal surface hyaline, thin- or slightly thick-walled, subparallel or ascending (hairs), some short-celled and slightly inflated, 3–7 μm in diam. Tramal hyphae hyaline, thin-walled, interwoven and rather loosely arranged, occasionally anastomosing, (2.2–) 2.3–4.8 (–5.0) μm in diam. (n = 80/4). Subhymenial hyphae hyaline, thin-walled, predominantly ascending, (1.8–) 1.9–3.3 (–3.5) μm in diam. (n = 80/4). Hyphidia abundant, occasionally branched, 0.8–1.2 μm in diam. at the apex, covering basidial cells or projecting above hymenial layer up to 25 μm. Basidia four-celled, (10.2–) 10.9–14.2 (–14.8) × (8.2–) 8.3–10.4 (–11.0) μm (n = 80/4), often arranged in a dense palisade and covering spines up to the very top, stalk up to 25 × 2–4 μm, sterigmata up to 28 × 2.5–3 μm. Basidiospores ellipsoid to broadly ellipsoid, more rarely subglobose, (6.0–) 6.1–7.9 (–8.2) × (5.0–) 5.1–6.7 (–6.9) μm (n = 120/4), L = 6.61–6.98, W = 5.48–6.11, Q′ = (1.0–) 1.1–1.4 (–1.5), Q = 1.14–1.22.
Distribution and ecology. Europe (Finland, Georgia, Russian Caucasus); fallen logs and branches of conifers (Abies nordmanniana, Picea orientalis, Pinus sylvestris, Thuja sp.).
Remarks. Pseudohydnum alienum is one of two representatives of the genus distributed in Europe. From another European species, P. gelatinosum, it differs in having smaller basidiocarps, distinctly narrower tramal and subhymenial hyphae, as well as slenderer hyphidia. In addition, subhymenial hyphae of P. alienum are more regularly packed, predominantly ascending, and basidia are normally arranged in a palisade manner. On the contrary, the subhymenial structure of P. gelatinosum looks much more disorderly, with hyphae mostly oriented in a random way. Differences of P. alienum from the phylogenetically close P. omnipavum and P. translucens are discussed under these species.
Pseudohydnum alienum is a poorly sampled species known from two collections from the Caucasus and two specimens from the southwestern Finland. One of the latter specimens was collected from remains of a cultivated Thuja tree. DNA sequences of the Finnish and Caucasian specimens are identical.
Specimens examined. Finland. Varsinaissuomi: Raasepori, Lökudden Nat. Res., Thuja sp. (fallen decorticated branch), 16.IX.2008 Kotiranta 22407* (H), Tjurberget, Pinus sylvestris (cut bolt), 6.XI.2021 Miettinen 25013 (H). Georgia. Imereti: Tqibuli, Kharistvali, Abies nordmanniana (fallen log), 18.X.1963 Parmasto (TAAM 015898). Russia. Karachay-Cherkessia (holotype, see above).
Pseudohydnum brunneiceps Y.L. Chen, M.S. Su & L.P. Zhang, Phytotaxa 441: 91, 2020. — Fig. 10
Holotype. China. Jiangxi: Jiujiang Co., Lushan Nat. Res., coniferous wood, 26.VI.2018 Zhang (JXSB0967).
Basidiocarps up to 3 cm in widest dimension and 2–3 mm thick, pileate, laterally stipitate, gelatinous. Pileal surface strigose, reddish-brownish to brown. Pileal edge sharp, fertile. Spines sharp-tipped, white to cream-coloured, 0.5–2 mm long, 4–5 per mm. Stipe up to 3 cm long and 5 mm in diam., equally thickened along the whole length, greyish- or reddish-brown, strigose or partly covered by spines.
Hyphal structure monomitic, hyphae clamped. Hyphae of pileal surface greyish or brownish, thin- or slightly thick-walled, subparallel or ascending (hairs), some short-celled and inflated, 2–8 μm in diam. Tramal hyphae hyaline to greyish or brownish, thin-walled, interwoven, occasionally anastomosing, (2.3–) 2.9–5.4 (–6.0) μm in diam. (n = 60/3). Subhymenial hyphae hyaline, thin-walled, interwoven, (2.1–) 2.2–3.3 (–3.6) μm in diam. (n = 20/1). Hyphidia abundant, richly branched, 1.5–2 μm in diam. at the apex, covering basidial cells. Basidia four-celled, predominantly obliquely septate, (10.2–) 10.8–12.8 (–13.3) × (7.0–) 7.2–8.4 (–8.8) μm (n = 30/1), scattered and embedded in masses of hyphidia, stalk up to 15 × 2.5–4 μm, sterigmata up to 17 × 2–2.5 μm. Basidiospores ellipsoid to broadly ellipsoid, rarely subglobose, (6.8–) 6.9–8.9 (–9.2) × (6.0–) 6.1–7.1 (–8.0) μm (n = 30/1), L = 7.76, W = 6.66, Q′ = (1.0–) 1.1–1.3 (–1.4), Q = 1.17.
Distribution and ecology. Southeast Asia (China — Hunan, Jiangxi); rotten wood of conifers.
Remarks. Dark-coloured basidiocarps with strigose upper surface and a well-developed stipe differentiate P. brunneiceps from all other species described here. Microscopically, large, broadly ellipsoid basidiospores and predominantly obliquely septate basidia are characteristic for the species. Pseudohydnum brunneiceps was introduced based on collections from Jiangxi, Southeast China, where it inhabits decayed wood of conifers, mainly Cryptomeria japonica (Chen et al. 2020). Here, we report it from Hunan.
Specimen examined. China. Hunan: Liu Yang Co., Daweishan Nat. Park Forest, fallen log, 28.IX.2000 Härkönen K884* (H).
Pseudohydnum cystidiatum V. Malysheva & V. Dudka, sp. nov. — Figs. 6, 9, and 10
MycoBank MB846387.
Holotype. Vietnam. Cao Bằng Province: Nguyên Bình Dist., Thành Công, 22.60695N 105.87303E, decayed wood, 10.IV.2021 Dudka (LE 313656*).
Etymology: cystidiatus (Lat., adj.) — bearing cystidia.
Basidiocarps up to 1 cm in widest dimension and 1–1.5 mm thick, pileate, sessile, gelatinous. Pileal surface papillate, pale ochraceous when fresh, greyish-brownish in dry condition. Pileal edge sharp, somewhat undulating, fertile. Spines sharp-tipped, white to cream-coloured, up to 0.5 mm long, 3–4 per mm. Stipe rudimentary, very short, or absent.
Hyphal structure monomitic, hyphae clamped. Hyphae of pileal surface hyaline, thin- or slightly thick-walled, subparallel, some short-celled and slightly inflated, 2–7 μm in diam. Tramal hyphae hyaline, thin-walled, interwoven, frequently anastomosing, (1.5–) 2.0–5.0 (–4.5) μm in diam. (n = 20/2), accidentally inflated (up to 6 μm in diam.) at septa, in uppermost layers hyaline and with a distinct wall. Subhymenial hyphae hyaline, thin-walled, interwoven, (1.0–) 1.2–2.0 (–2.3) μm in diam. (n = 20/2). Hyphidia abundant, occasionally branched, 1–1.5 μm in diam. at the apex, covering basidial cells. Cystidia hyaline, rather rare, tapering, projecting above hymenial layer, (27–) 27.2–43 (–58) × (11.5–) 15.5–16.7 (–18.0) μm (n = 6/2). Basidia four-celled, (10.1–) 10.5–12.9 (–13.1) × (8.0–) 8.9–10.2 (–10.6) μm (n = 20/2), scattered and mostly embedded in masses of hyphidia, stalk up to 48 × 2–2.5 μm, sterigmata up to 30 × 2–3 μm. Basidiospores broadly ellipsoid to subglobose, (6.6–) 6.8–9.5 (–9.6) × (4.9–) 5.6–7.6 (–8.1) μm (n = 30/1), L = 8.10, W = 6.38, Q′ = 1.1–1.5 (–1.6), Q = 1.27.
Distribution and ecology. Southeast Asia (Vietnam); decayed wood in lowland evergreen mixed forest.
Remarks. The presence of hymenial cystidia differentiate P. cystidiatum from other species described in the present paper. From two other Vietnamese species (P. meridianum and P. placibile) described here, P. cystidiatum differs in having small basidiocarps with a rudimentary stipe and larger, broadly ellipsoid basidiospores. The species is so far known only from two localities in Vietnam.
Specimens examined. Vietnam. Cao Bằng Province: Nguyên Bình Dist., Thành Công, 10.IV.2021 Dudka (holotype, see above), National Park Phia Oac–Phia Den, decayed wood, 15.IV.2021 Dudka (LE 313657*).
Pseudohydnum gelatinosum (Scop.) P. Karst., Not. Sällsk. Fauna Flora Fennica Förh. 9: 374, 1868. — Figs. 7, 9, and 10
≡ Hydnum gelatinosum Scop., Flora Carniolica 2: 472, 1772. Lectotype. Table 9 (p. 239) in Jacquin, Miscellanea Austriaca ad Botanicam, Chemiam et Historiam Naturalem Spectantia 1, 1778 (designated here, MBT10010097). Epitype. Slovenia. Idrija: Idrija, 45.992E 14.017N, Picea abies (old cut stump), 27.IX.2019 Grebenc & Spirin 13369* (LJF, dupl. H 7200586 and LE) (designated here, MBT10010098).
= Hydnum crystallinum O.F. Müll., Flora Danica 4 (12): 6, 1777. Lectotype. Figure 717 in Müller, op. cit. (designated here, MBT10010099).
= Hydnum clandestinum Batsch, Elenchus Fungorum: 113, 1783. Lectotype. Figure 44 (Table X) in Batsch, op. cit. (designated here, MBT10010100).
Basidiocarps rarely exceeding 4 cm in widest dimension, 2–4 mm thick, pileate, laterally stipitate, gelatinous. Pileal surface first strigose, watery-greyish or greyish-brownish, then almost smooth, grey to brown, pale ochraceous or greyish-brownish to dark brown in dry condition. Pileal edge sharp to rather blunt, fertile. Spines sharp-tipped, white to greyish, 1–3 mm long, 5–8 per mm. Stipe up to 1 cm long (rarely longer), watery-greyish, usually covered by spines up to the very base, in some specimens rudimentary.
Hyphal structure monomitic, hyphae clamped. Hyphae of pileal surface hyaline or greyish, thin- or slightly thick-walled, subparallel or ascending (hairs), 3–10 μm in diam., some short-celled and inflated up to 16 μm. Tramal hyphae hyaline or greyish, thin-walled, interwoven, occasionally anastomosing, (1.7–) 1.9–9.2 (–9.7) μm in diam. (n = 140/7), accidentally inflated (up to 12 μm in diam.) at septa. Subhymenial hyphae hyaline or greyish, thin-walled, interwoven, (1.9–) 2.0–4.3 (–4.6) μm in diam. (n = 140/7). Hyphidia abundant, occasionally branched, 1–2.5 μm in diam. at the apex, scattered among basidial cells or partly covering them, sometimes projecting above hymenial layer up to 40 μm. Basidia four-celled, (8.8–) 9.8–14.4 (–14.8) × (7.0–) 7.4–10.9 (–11.2) μm (n = 160/8), arranged in a dense palisade or scattered among hyphidia, stalk up to 35 × 2–3 μm, sterigmata up to 25 × 2–3 μm. Sterile spine tips up to 200 μm long. Basidiospores ellipsoid to broadly ellipsoid, (5.2–) 5.3–9.0 (–9.2) × (4.6–) 4.9–7.6 (–8.2) μm (n = 690/23), L = 6.02–7.67, W = 5.11–6.61, Q′ = (1.0–) 1.1–1.5 (–1.6), Q = 1.09–1.28.
Distribution and ecology. Europe, Asia (Ural, Siberia, Russian Far East); various wood remnants of conifers.
Remarks. Hydnum gelatinosum was described by Scopoli (1772) from the present-day Slovenia, and the name was subsequently sanctioned by Fries (1821: 407). No authentic material of H. gelatinosum survives except Scopoli’s oil painting stored in the National Museum of Natural History, Paris (A. Piltvater, pers. comm. 13.XII.2020). However, we see no strong reasons to designate this illustration as a lectotype of H. gelatinosum. First, there is no reference to the oil painting (or any other illustrative material) in the protologue. Second, Scopoli’s oil paintings have a formal status of copyright material, and therefore, they are currently not available for a broader audience. The current version of the Code allows us to select a lectotype from the sanctioning treatment, i.e., Fries’ Systema Mycologicum (1821). Therein, Fries referred to a colour figure of H. gelatinosum by Jacquin (1778); this book is available online and can be easily accessed by researchers. We designate the latter illustration as a lectotype (iconotype) of H. gelatinosum. Additionally, a recent sequenced specimen of P. gelatinosum from Idrija (Slovenia), the place where Scopoli lived and collected many of his species, is selected as epitype (see above).
Fries (1821) mentioned Hydnum crystallinum O.F. Müll. (described from Denmark) and H. clandestinum Batsch (described from Germany, presumably Thuringia) under synonyms of H. gelatinosum. No original specimens exist for either of them but illustrations are available (Müller 1777; Batsch 1783), and we use them for lectotypification of these taxa. In both cases, distinct brownish coloration of the pileal surface was depicted: this feature certainly refers to P. gelatinosum as redefined here and rules out another European species, P. alienum (see description above). Donk (1966) treated H. auriculatum Fr. as one more synonym of P. gelatinosum. The protologue (Fries 1838) seemingly refers to P. gelatinosum. The single specimen of H. auriculatum in Fries’ herbarium (UPS F-117034) consists of two pieces with different labels (Å. Kruys, pers. comm. 19.IV.2023). Unfortunately, it is impossible to decide whether they were collected before 1838. Buxbaum’s illustration cited by Fries in the protologue of H. auriculatum makes the idea of this species even more obscure. This illustration (Buxbaum 1728: Table 56; Fig. 2) is almost incomprehensible, and the accompanying description (as “Agaricus gelatinosus, parte prona erinaceus,” p. 36) refers to a hydnoid fungus with bluish or light-violet fructifications growing on wood of Alnus (“dilute coeruleus aut purpurascens, antiquaram Alnorum truncis adnascitur,” ibid.). These indications are certainly at odds with Fries’s own description of H. auriculatum (“pileis … murinis, aculeis teretibus brevibus albis… In truncis Pini” — Fries, op. cit., p. 513). At the moment, we leave this problem unresolved.
Bourdot and Galzin (1927) introduced Tremellodon crystallinum var. exidiodon as a taxon deviating from P. gelatinosum (treated by them as T. crystallinum (O.F. Müll.) Quél.) due to its globose basidiocarps and a peculiar host (Populus). This variety was even suggested to represent a separate species (Pilát 1957) although never formally raised to the species rank. We studied the single authentic collection of T. crystallinum var. exidiodon (Aveyron, Crouzette, 15.XII.1913 Galzin 14918 (herb. Bourdot 12384, PC)) and concluded it shows no morphological differences from typical specimens of P. gelatinosum. However, we are unaware of other collections of this species from deciduous trees. Newly collected material from the southern part of France is highly desirable for settling taxonomic status of this variety.
Pseudohydnum gelatinosum is the most common representative of the genus in temperate–boreal forests of Eurasia. Morphologically, it is a highly diverse species, and its recognition versus sister taxa demands a meticulous microscopic study. The variation range of basidiospores in P. gelatinosum is huge, and it covers the variation range of all other temperate–boreal species except P. umbrosum (Fig. 11). Therefore, P. gelatinosum can be confidently distinguished from P. alienum in Europe, P. translucens in Asia, and P. omnipavum in North America due to distinctly wider tramal hyphae and hyphidia (Table 2). The North-American collections of P. gelatinosum are described below as P. gelatinosum ssp. pusillum.
Specimens examined. Belarus. Minsk Reg.: Borisov Dist., Kischino Sloboda, Picea abies, 20.VIII.1934 Nikolaeva (LE 38784*). Finland. Uusimaa: Helsinki, Puotila, Juorumäki, P. abies, 13.IX.1999 Heikurainen* (H), Veräjämäki, P. abies, 6.XII.2015 Miettinen 19872.2* (H); Sipoo, Pilvijärvi, P. abies, 4.X.2011 Pekanpalo* (H). Pohjois-Karjala: Lieksa, Koli Nat. Park, P. abies, 13.IX.2011 Härkönen 32* (H). Satakunta: Ylöjärvi, Inkula, P. abies, 10.IX.2014 Niemelä 9164* (H). Perä-Pohjanmaa: Rovaniemi, Pisavaara, P. abies, 15.VIII.2009 Kinnunen 5242 (H). Kainuu: Puolanka, Paljakka Strict Nat. Res., P. abies, 29.IX.2018 Miettinen 21866* (H). Poland. Podlesie: Hajnówka Dist., Białowieża Primaeval Forest, P. abies, 10.IX.2009 Schigel 6434* (H). Russia. Altai Rep.: Ulagan Dist., Altai Nat. Res., Atkichu, decayed coniferous wood, 15.VIII.2008 Psurtseva (LE 265229*). Bashkortostan: Beloretsk Dist., Bashkirsky Nat. Res., Ural-Tau, Larix sibirica, 30.VIII.1945 Selivanova (LE 38780*). Irkutsk Reg.: Ust’-Kut Dist., Orlenga, L. sibirica, 8.IX.1967 Bondartseva (LE 38791*). Khakassia: Abakan, Erkagi, Picea obovata, 11.VIII.2011 Kotiranta 23028* (H). Krasnoyarsk Reg.: Ermakovskoe Dist., Sayano-Shushensky Nat. Res., Talovka, Picea sp., 8.VIII.2020 V. Malysheva (LE 313583*), 9.VIII.2020 Kiyashko (LE 313584*), Bol’shaya Golaya, Abies sibirica, 24.VIII.2020 V. Malysheva (LE 313587*); Turukhansk Dist., Lebed’, P. obovata, 23.VIII.2013 Kotiranta 26434* (H). Leningrad Reg.: Boksitogorsk Dist., Kolp’, P. abies, 27.VII.2016 Spirin 10388* (H); Kirishi Dist., Shariya, P. abies, 11.VIII.2019 Spirin 12948* (H); Luga Dist., Natalino, P. abies, 4.X.2015 Volobuev (LE 313779*); Tosno Dist., P. abies, 20.VIII.1999 Morozova (LE 208530*). Moscow Reg.: Krasnogorsk Dist., Opalikha, Pinus sylvestris, 26.IX.2017 Matershev (LE 315333*). Primorie: Shkotovo Dist., Anisimovka, rotten wood, 31.VIII.2001 Diakov (LE 313661*, 313662*). Pskov Reg.: Loknya Dist., Bashovo, P. sylvestris, 1.VII.1998 Popov (LE 222719*). Sakhalin Reg.: Kunashir, Yuzhno-Kuril’sk, rotten wood, 10.IX.1989 Kovalenko (LE 313565*), Tretyakova River, rotten log, 15.VIII.2017 Bulakh (LE 313567*). Slovenia. Jesenice: Mojstrana, Triglavska Bistrica, P. abies, 28.IX.2019 Grebenc & Spirin* 13446 (H, LJF). Kočevje: Rajhenav, Abies alba, 30.VII.2020 Grebenc & Spirin 14001* (H). Radovljica: Bohinj, Mrežce, P. abies, 26.IX.2019 Spirin 13196*, 13198, 13211* (H, LJF). Sweden. Bohuslän: Hjärtum, Valdalen, P. abies, 27.IX.1974 Jeppson 900 (GB-0185577). Halland: Fjärås, Bräckan, coniferous wood, 10.X.1968 Karlvall 13227 (GB-0185571); Halmstad, Biskopstorps Nat. Res., P. abies, 28.IX.2012 Schigel 7414 (GB-0130727). Småland: Rumskulla, Norra Kvill Nat. Park, P. abies, 8.X.1966 Eriksson (GB-0185570). Västmanland: Viker, Sjöändan, coniferous wood, 29.VIII.1981 Jeppson 2061 (GB-0185595).
Pseudohydnum gelatinosum ssp. pusillum (Ellis & Everh.) Miettinen & Viner, comb. nov. — Figs. 8 and 10
Anatomical structures of Pseudohydnum spp.: 1 — P. alienum (holotype): a — basidia, hyphidia, subhymenial hyphae; b — tramal hyphae; c — hyphae of pileal surface; 2 — P. cystidiatum (holotype): a — basidia, hyphidia, subhymenial hyphae; b — tramal hyphae; c — cystidia; 3 — P. gelatinosum (epitype): a — basidia, hyphidia, subhymenial hyphae; b — tramal hyphae; c — hyphae of pileal surface; 4 — P. placibile (holotype): a — basidia, hyphidia, subhymenial hyphae; b — tramal hyphae. Scale bar = 10 μm
MycoBank MB846388.
≡ Tremellodon pusillus Ellis & Everh., Proc. Acad. Nat. Sci. Philadelphia 46: 323, 1894. Neotype. USA. Washington: Jefferson Co., Hoh River, 47.86417N 123.92826W, Tsuga heterophylla (fallen tree crown), 20.X.2014 Miettinen 18987.2* (H 7200192) (designated here, MBT10010101).
Basidiocarps up to 5 cm in widest dimension and 1–3 mm thick, pileate, laterally stipitate, gelatinous. Pileal surface first strigose, watery-greyish or greyish-brownish, then almost smooth, brown, pale ochraceous or greyish-brownish in dry condition. Pileal edge sharp to rather blunt, fertile. Spines sharp-tipped, white to greyish, 1–3 mm long, 6–8 per mm. Stipe up to 0.5 cm long, watery-greyish, usually covered by spines up to the very base, sometimes rudimentary.
Hyphal structure monomitic, hyphae clamped. Hyphae of pileal surface hyaline or greyish, thin- or slightly thick-walled walled, subparallel or ascending (hairs), some short-celled and inflated, 3–12 μm in diam. Tramal hyphae hyaline or greyish, thin-walled or with a distinct wall, subparallel to interwoven, occasionally anastomosing, (2.9–) 3.0–8.2 (–9.0) μm in diam. (n = 100/5), accidentally inflated (up to 10 μm in diam.) at septa. Subhymenial hyphae hyaline or greyish, thin- or slightly thick-walled, interwoven or ascending, (2.0–) 2.1–4.0 (–4.2) μm in diam. (n = 100/5). Hyphidia abundant, occasionally branched, 1–2 μm in diam. at the apex, partly covering basidial cells or projecting up to 20 μm. Basidia four-celled, (10.4–) 10.6–14.5 (–15.2) × (8.0–) 8.1–11.1 (–12.8) μm (n = 100/5), scattered among hyphidia, stalk up to 32 × 3–3.5 μm, sterigmata up to 25 × 2–3 μm. Sterile spine tips up to 150 μm long. Basidiospores broadly ellipsoid to subglobose, (6.0–) 6.1–7.3 (–7.6) × (5.1–) 5.2–6.7 (–6.8) μm (n = 150/5), L = 6.62–6.90, W = 5.65–6.04, Q’ = 1.0–1.3, Q = 1.10–1.21.
Distribution and ecology. North America (USA – New York, North Carolina, Tennessee, Washington); fallen logs of conifers.
Remarks. Tremellodon pusillus was described from the Olympic Peninsula, the northwestern part of the USA (Ellis & Everhart 1894). The species quickly became forgotten, most likely because of the lack of the surviving type material. The single specimen (a presumable type) was cited in the protologue. We could not trace this collection in the contemporary public herbaria and thus consider it lost. Therefore, the only remaining source for understanding of T. pusillus is the original description. Ellis and Everhart described their species as having smoky-brown upper surface with sparse hairs and producing a rather long (up to 1 cm) stipe. These features preclude P. omnipavum (see description below) distributed in the same geographic region but fit well to the North-American subspecies of P. gelatinosum (henceforth treated as P. gelatinosum ssp. pusillum). A neotype for T. pusillus from the Olympic Peninsula is selected here to support our viewpoint.
Pseudohydnum gelatinosum spp. pusillum normally produces somewhat larger and darker basidiocarps than P. gelatinosum s. str. from Eurasia. Its basidiospores are not so variable as in the latter taxon; nevertheless, their dimensions totally fall within the range limits of P. gelatinosum s. str. No reliable anatomical traits were detected by us to distinguish these two taxa, and P. gelatinosum ssp. pusillum is treated here as a separate entity mainly because of its small genetic differences from P. gelatinosum s. str. Further studies with the use of additional markers may provide better interpretation of P. gelatinosum ssp. pusillum.
Specimens examined. USA. New York: Essex Co., Huntington Wildlife Forest, rotten wood, 16.VIII.2012 Sjökvist (H), Picea rubens, 17.VIII.2012 Miettinen 15646 (H), Tsuga canadensis (?), 16.IX.2013 Miettinen 16888 (H), snag (Tsuga or Picea), 17.IX.2013 Miettinen 16894* (H); Hamilton, Huntington Wildlife Forest, T. canadensis, 19.IX.2013 Miettinen 17010 (H). North Carolina: Swain Co., Clingmans Dome, Abies fraseri/Picea rubens, 1.X.2015 Miettinen 19625.1* (H). Tennessee: Cocke Co., Cosby Creek, Tsuga canadensis, 2.X.2015 Miettinen 19671* (H). Washington (neotype of T. pusillus, see above).
Pseudohydnum meridianum V. Malysheva & Spirin, sp. nov. — Figs. 6 and 12
MycoBank MB846389.
Holotype. Vietnam. Gia Lai Province: K’Bang Dist., Son Lang, Kon Chu Rang Nature Reserve, 14.51805N 108.55376E, decayed wood, 31.V.2016 Morozova (LE 313568*, isotype – H 7200587).
Etymology: meridianus (Lat., adj.) – southern.
Basidiocarps up to 4 cm in widest dimension and 1–2 mm thick, pileate, laterally stipitate, gelatinous. Pileal surface papillate or smooth, greyish-brown when fresh, dark brown to almost black in dry condition. Pileal edge sharp, somewhat undulating, fertile. Spines sharp-tipped, white to cream-coloured, 0.5–1 mm long, 3–4 per mm. Stipe up to 2 cm long and 5 mm in diam., gradually tapering to the base, concolorous with pileal surface, finely tomentose to almost smooth.
Hyphal structure monomitic, hyphae clamped. Hyphae of pileal surface hyaline or greyish, thin- or slightly thick-walled, subparallel or ascending (hairs), some short-celled and slightly inflated, 3–10 μm in diam. Tramal hyphae hyaline, thin-walled, interwoven, frequently anastomosing, (2.0–) 2.8–7.2 (–7.3) μm in diam. (n = 20/1), accidentally inflated (up to 9 μm in diam.) at septa, in uppermost layers greyish and with a distinct wall. Subhymenial hyphae hyaline, thin-walled, interwoven, (1.8–) 1.9–2.7 (–2.8) μm in diam. (n = 20/1). Hyphidia abundant, occasionally branched, 1–1.5 μm in diam. at the apex, covering basidial cells. Basidia four-celled, (10.3–) 10.7–13.0 (–13.2) × (7.8–) 8.0–10.3 (–10.4) μm (n = 20/1), scattered and mostly embedded in masses of hyphidia, stalk up to 25 × 2–2.5 μm, sterigmata up to 30 × 2–3 μm. Sterile spine tips up to 120 μm long. Basidiospores subglobose to globose, rarely broadly ellipsoid, (5.0–) 5.1–6.2 (–6.3) × (4.8–) 4.9–5.8 (–6.0) μm (n = 30/1), L = 5.65, W = 5.26, Q’ = 1.0–1.1 (–1.2), Q = 1.07.
Distribution and ecology. Southeast Asia (Vietnam); decayed wood in lowland evergreen mixed forest.
Remarks. Dark-coloured basidiocarps with a pronounced stipe and nearly smooth pileal surface, as well as small, predominantly subglobose basidiospores differentiate P. meridianum from other species distributed in the Southeast Asia. The species is so far known from a few localities in Vietnam.
Specimens examined. Vietnam. Gia Lai Province: K’Bang Dist., Son Lang, Kon Chu Rang Nature Reserve, decayed wood, 24.X.2022 Dudka (LE F-347479*, LE F-347480*), 27.X.2022 Dudka (LE F-347478*). Dak Nong Province, Dak Glong District, Ta Dung National Park, decayed wood, 11.X.2022 Dudka (LE F-347477*).
Pseudohydnum omnipavum Spirin & Miettinen, sp. nov. — Figs. 8 and 12
MycoBank MB846390.
Holotype. USA. Idaho: Boundary Co., Upper Priest River, 48.927N 117.333W, Pseudotsuga menziesii (fallen partly corticated log), 16.X.2014 Spirin 8667* (H 7200588, isotype – LE).
Etymology: omnipavus (Lat., adj.) — trembling.
Basidiocarps up to 2 cm in widest dimension and 1–2 mm thick, pileate, laterally stipitate, gelatinous. Pileal surface finely tomentose to strigose, first watery-greyish, then greyish, pale ochraceous or greyish-brownish in dry condition. Pileal edge sharp, fertile. Spines sharp-tipped, white to cream-coloured, 0.5–1.5 mm long, 4–6 per mm. Stipe up to 0.5 cm long, watery-greyish, finely tomentose or more or less smooth and covered by spines.
Hyphal structure monomitic, hyphae clamped. Hyphae of pileal surface hyaline, thin-walled, subparallel or ascending (hairs), some short-celled and inflated, 3–9 μm in diam. Tramal hyphae hyaline, thin-walled, interwoven, occasionally anastomosing, (2.8–) 2.9–4.2 (–4.3) μm in diam. (n = 40/2), in some parts tightly glued by gelatinous matter. Subhymenial hyphae hyaline, thin-walled, interwoven, (2.0–) 2.1–3.3 (–3.8) μm in diam. (n = 40/2). Hyphidia abundant, richly branched, 1–1.5 μm in diam. at the apex, partly covering basidial cells. Basidia four-celled, (9.8–) 9.9–12.2 (–12.3) × (7.0–) 7.8–10.1 (–10.2) μm (n = 40/2), scattered among hyphidia and covering spines up to the very top, stalk up to 30 × 2–3 μm, sterigmata up to 20 × 2–3 μm. Basidiospores broadly ellipsoid to subglobose, rarely globose, (5.2–) 5.8–7.3 (–7.6) × (4.8–) 5.1–6.2 (–7.2) μm (n = 60/2), L = 6.39–6.55, W = 5.67–5.87, Q′ = 1.0–1.2 (–1.4), Q = 1.09–1.16.
Distribution and ecology. North America (USA–Idaho, Canada–British Columbia); fallen logs of conifers (Pseudotsuga, Tsuga).
Remarks. Phylogenetically and morphologically, P. omnipavum is closest to P. alienum and P. translucens. These three species possess narrow tramal hyphae and slender hyphidia. From the latter two species, P. omnipavum can be distinguished mainly due to a better-differentiated stipe and a lesser number of spines per mm, as well as different distribution area (North America vs. Eurasia). The species is so far known from two localities in Rocky Mts.
Specimens examined. Canada. British Columbia: Columbia — Shuswap Regional Dist., Glacier Nat. Park, fallen coniferous log, 24.VIII.1982 Hallenberg 6817 (GB-0185596). USA. Idaho: Boundary Co., Upper Priest River, Tsuga heterophylla (fallen tree crown), 16.X.2014 Miettinen 18877* (H).
Pseudohydnum placibile V. Malysheva & V. Dudka, sp. nov. — Figs. 6, 9, and 12
MycoBank MB846391.
Holotype. Vietnam. Cao Bằng Province: Nguyên Bình Dist., Thành Công, National Park Phia Oac — Phia Den, 22.60661N 105.87346E, decayed wood, 15.IV.2021 Do & Dudka (LE 313658*).
Etymology: placibilis (Lat., adj.) – pleasant, attractive.
Basidiocarps up to 1.5 cm in widest dimension and 1–1.5 mm thick, pileate, stipitate, gelatinous. Pileal surface papillate, pale ochraceous when fresh, greyish-brownish in dry condition. Pileal edge sharp, somewhat undulating, fertile. Spines sharp-tipped, white to cream-coloured, up to 0.5 cm long, 2–3 per mm. Stipe up to 1 cm long, watery-greyish, usually covered by spines up to the very base.
Hyphal structure monomitic, hyphae clamped. Hyphae of pileal surface hyaline, thin- or slightly thick-walled, subparallel, some short-celled and slightly inflated, 2–5 μm in diam. Tramal hyphae hyaline, thin-walled, rare interwoven, frequently anastomosing, (1.0–) 1.5–2.5 (–4.0) μm in diam. (n = 20/2), accidentally inflated (up to 5 μm in diam.) at septa, in uppermost layers hyaline and with a distinct wall. Subhymenial hyphae hyaline, thin-walled, interwoven, (1.0–) 1.2–1.5 (–2.0) μm in diam. (n = 20/2). Hyphidia abundant, occasionally branched, 1–1.5 μm in diam. at the apex, covering basidial cells. Basidia four-celled, (9.5–) 10.1–11.4 (–12.0) × (7.5–) 7.6–9.3 (–9.6) μm (n = 20/2), scattered and mostly embedded in masses of hyphidia, stalk up to 45 × 2–2.5 μm, sterigmata up to 25 × 2–3 μm. Basidiospores subglobose to globose, rarely broadly ellipsoid, (5.3–) 5.4–6.4 × (3.7–) 4.1–5.1 (–5.4) μm (n = 30/1), L = 5.89, W = 4.69, Q′ = (1.1–) 1.2–1.4 (–1.5), Q = 1.26.
Distribution and ecology. Southeast Asia (Vietnam); decayed wood in lowland evergreen mixed forest.
Remarks. The absence of hairs on pileal surface and relatively small, predominantly subglobose basidiospores make P. placibile similar to another Southeast Asian species, P. meridianum. Nevertheless, P. placibile can be differentiated from P. meridianum due to much paler basidiocarps and distinctly narrower tramal hyphae. The species is so far known from two collections in the type locality (Vietnam).
Specimens examined. Vietnam. Cao Bằng Province: Nguyên Bình Dist., Thành Công, National Park Phia Oac — Phia Den, decayed wood, 7.IV.2021 Dudka (LE 313659*), 15.IV.2021 Do & Dudka (holotype, see above).
Pseudohydnum translucens Lloyd, Mycological Writings 7: 1357, 1925. — Figs. 8 and 12
Holotype. Japan. Kansai: Hyōgo, Kobe, [no collecting date] Lewis (BPI 0324962, studied).
= Pseudohydnum candidissimum H.M. Zhou, T. Bau & Jing Si, Frontiers in Cellular and Infection Microbiology 13:1139449: 5, 2023.
Basidiocarps up to 2 cm in widest dimension and 1–2 mm thick, pileate, laterally stipitate, gelatinous. Pileal surface first strigose, watery-greyish, then almost smooth, greyish-brownish, pale ochraceous or greyish-brownish in dry condition. Pileal edge sharp to rather blunt, fertile. Spines sharp-tipped, white to greyish, 1–1.5 mm long, 6–8 per mm. Stipe up to 0.5 cm long, watery-greyish, usually covered by spines up to the very base.
Hyphal structure monomitic, hyphae clamped. Hyphae of pileal surface hyaline, thin- or slightly thick-walled, subparallel or ascending (hairs), some short-celled and slightly inflated, 2–9 μm in diam. Tramal hyphae hyaline, thin-walled, interwoven, occasionally anastomosing, (1.1–) 1.4–5.2 (–5.4) μm in diam. (n = 160/8), accidentally inflated (up to 6.5 μm in diam.) at septa. Subhymenial hyphae hyaline, thin-walled, mostly ascending, (1.0–) 1.1–3.0 (–3.2) μm in diam. (n = 140/7). Hyphidia abundant, occasionally branched, 1–2 μm in diam. at the apex, covering basidial cells. Basidia four-celled, (9.3–) 9.8–13.2 (–13.5) × (8.2–) 8.3–10.4 (–11.2) μm (n = 150/8), scattered and embedded in masses of hyphidia, stalk up to 35 × 2–3 μm, sterigmata up to 20 × 2–3 μm. Sterile spine tips up to 150 μm long. Basidiospores ellipsoid to ovoid or subglobose, (5.3–) 5.4–7.4 (–8.0) × (4.1–) 4.2–6.9 (–7.0) μm (n = 240/8), L = 6.24–6.85, W = 5.01–5.66, Q′ = (1.0–) 1.1–1.5 (–1.7), Q = 1.12–1.29.
Distribution and ecology. East Asia (China, Japan, Siberia, Russian Far East); fallen logs and branches of conifers (predominantly Abies spp.).
Remarks. Pseudohydnum translucens is the East Asian relative of P. alienum. The latter species is distributed in Europe and it differs from P. translucens in having on average wider basidiospores and narrower hyphidia (Table 2). Moreover, basidia of P. translucens are intermixed with hyphidia in a highly irregular manner, while they tend to be arranged in a dense palisade layer in P. alienum. Two other Pseudohydnum species distributed in the same geographic area, P. gelatinosum and P. umbrosum, have more robust basidiocarps with more intensively coloured, grey to brownish-black upper surface, as well as wider hyphae and larger basidiospores.
Specimens examined. Japan. Kansai (holotype, see above). Russia. Jewish Autonomous Reg.: Obluchensky Dist., Yadrino, fallen coniferous log, 10.VIII.1961 Raitviir (TAAM 042211). Krasnoyarsk Reg.: Ermakovskoe Dist., Sayano-Shushensky Nat. Res., Talovka, Abies sibirica, 7–8.VIII.2020 V. Malysheva (LE 313582*, LE 313585*, 313586*). Primorie. Terney Dist.: Sikhote-Alin Nat. Res., Maisa, strongly decayed log, 21.VIII.1996 Morozova (LE 262798*). Sakhalin Reg.: Kunashir, Goryachii Plyazh, Abies sachalinensis (fallen branch), 2.X.1960 Parmasto (TAAM 012200); Nevel’sky Dist., A. sachalinensis (fallen branch), 12.IX.1979 Parmasto (TAAM 102447).
Pseudohydnum umbrosum V. Malysheva & Spirin, sp. nov. — Figs. 8 and 12
MycoBank MB846392.
Holotype. Russia. Krasnoyarsk Reg.: Ermakovskoe Dist., Sayano-Shushensky Nat. Res., 2 km upstream of the Bol’shaya Golaya River, 52.56388N 92.12777E, rotten coniferous log, 15.VIII.2015 V. Malysheva (LE 312767*, isotype – H 7200589).
Etymology: umbrosus (Lat., adj.) – dark-coloured.
Basidiocarps up to 2 cm in widest dimension and 1–2 mm thick, pileate, sessile, gelatinous. Pileal surface strigose, greyish-brown when fresh, brown to brownish-black in dry condition. Pileal edge rather blunt, fertile. Spines sharp-tipped, white to greyish, 0.5–2 mm long, 5–7 per mm. Stipe rudimentary, very short, or absent.
Hyphal structure monomitic, hyphae clamped. Hyphae of pileal surface hyaline to brownish, thin- or slightly thick-walled, subparallel or ascending (hairs), densely glued together and mostly hardly discernible, some short-celled and inflated, 3–8 μm in diam. Tramal hyphae hyaline, thin-walled, interwoven, occasionally anastomosing, (3.0–) 3.4–7.6 (–8.7) μm in diam. (n = 40/2), accidentally inflated (up to 14 μm in diam.) at septa. Subhymenial hyphae hyaline, thin-walled, ascending or interwoven, (2.0–) 2.1–4.2 (–4.8) μm in diam. (n = 40/2). Hyphidia present, sparsely branched, 1.5–2 μm in diam. at the apex. Basidia four-celled, (12.0–) 12.2–15.8 (–16.2) × (9.0–) 9.1–12.2 (–12.8) μm (n = 40/2), openly and loosely arranged, usually covering spines up to the very top, stalk up to 50 × 3–3.5 μm, sterigmata up to 25 × 2–3 μm. Basidiospores narrowly to broadly ellipsoid, (7.1–) 7.6–9.8 (–10.2) × (5.4–) 6.0–7.1 (–7.3) μm (n = 60/2), L = 8.47–8.79, W = 6.32–6.45, Q′ = (1.1–) 1.2–1.5 (–1.6), Q = 1.34–1.36.
Distribution and ecology. East Asia (Siberia, Russian Far East); decayed wood of conifers.
Remarks. Of the temperate–boreal species treated here, P. umbrosum is morphologically most distinctive due to a dark-coloured, fuscous-brown upper surface of pilei covered by short hairs. In addition, it possesses the largest spores in the genus; they are more or less regularly ellipsoid and have highest Q values comparing to other Pseudohydnum species.
Specimens examined. Russia. Krasnoyarsk Reg. (holotype, see above). Primorie: Khasan Dist., Kedrovaya Pad’ Nat. Res., decayed wood, 18.IX.1961 Parmasto (TAAM 015358).
Discussion
In the present study, we re-established identity of P. gelatinosum and described six new Pseudohydnum species from Eurasia and North America, in addition to eight species previously introduced by Chen et al. (2020) and Zhou et al. (2022, 2023). However, the species diversity in the genus seems to be much higher, as we could judge from the already existing data. Descriptions of P. gelatinosum s. lato from the neotropics provided by Möller (1895) and Lowy (1971) do not fit to any of the species treated here, likely referring to species yet to be described. Future taxonomic studies of the genus outside Europe and boreal North America should carefully reconsider already existing older names associated with P. gelatinosum, e.g. Hydnum hirneoloides Berk. & M.A. Curtis from Cuba.
The species concepts advocated above have been based on combined morphological and DNA evidence. The main obstacle for morphological definition of Pseudohydnum spp. is the large variability of basidiospores in the most common species, P. gelatinosum. Therefore, we propose to pay attention to other morphological traits for introducing new species. Among macroscopic characters, the pileal surface, basidiocarp colour(s), and a presence of stipe are the most important. Species-specific microscopic traits are the width of contextual and tramal hyphae, shape, and width of hyphidia and dimensions of basidia. Combined, these features provide a comprehensive background for the species delimitation. However, we cannot preclude an existence of morphologically indistinguishable Pseudohydnum species which could be identified via DNA tools only.
Of four generic markers used in this study, ITS, nc LSU rDNA, and to a lesser extent, RPB1 sequences allow of species recognition. The large divergence of TEF1 detected in P. gelatinosum deserves further investigations. Intraspecific variation of TEF1 is higher in this complex than interspecific variation of some other species, and phylogenetic signal is also mixed, placing some TEF1 copies far outside of the core P. gelatinosum clade (Fig. 5). Our previous studies of the Auriculariales (Spirin et al. 2018, 2019, 2020, 2021) revealed no conflicts between ITS–LSU- and TEF1-based phylogenies. Therefore, Pseudohydnum is the first documented case of obvious discordance between these markers. Among the Auriculariales, an unnamed Auricularia species was shown to have multiple copies of TEF1 region (Matheny et al. 2007). Further studies are needed to understand whether Pseudohydnum represents the same case.
Data on geographic distribution of most Pseudohydnum spp. remain rather fragmentary. Four species, P. brunneiceps, P. cystidiatum, P. meridianum, and P. placibile, seem to have a subtropical–tropical distribution in East and Southeast Asia; however, they all are known from a few records only. The rest of species are distributed in temperate–boreal forests of the northern hemisphere. Of them, P. gelatinosum is the most common Eurasian species, occurring on various wood remains of (almost exclusively) coniferous trees. Pseudohydnum translucens is seemingly restricted to East Asia, predominantly inhabiting remnants of Abies spp. Its closest relative, P. alienum, was so far found in Finland and Caucasus although it surely has a wider distribution. Therefore, much denser sampling of jelly fungi even in Europe is highly desirable.
The existence of two Pseudohydnum species in Europe made it necessary to collect the epitype material in the locus classicus of P. gelatinosum (Idrija, Slovenia). Although fulfilling minimal requirements for solid epitypification, re-collecting specimens in the type locality should consider changes of forest types in the targeted area. The Norway spruce (Picea abies) is currently the most common coniferous tree in the type locality of P. gelatinosum. In Scopoli’s time, the dominating tree species was evidently Abies alba. Additional sampling in the intact fir-dominated forest in Slovenia and sequencing this material for four markers confirmed that it is identical with our specimen of P. gelatinosum from the type locality, thus validating designation of the latter collection as an epitype of this species.
Data availability
DNA sequences used in the present study are available in GenBank. Alignments were deposited in PlutoF (https://doi.plutof.ut.ee/doi/10.15156/BIO/2912106). Fungal specimens are stored in public herbaria (as indicated under Specimens examined).
References
Batsch AJGC (1783) Elenchus fungorum. Jacob Gebauer, Magdeburg
Bourdot H, Galzin A (1927) Hyménomycètes de France. Hetérobasidiés – Homobasidiés gymnocarpes. Sceaux
Buxbaum JC (1728) Plantarum minus cognitarum Centuria I. Academia, St. Petersburg
Chen YL, Su MS, Zhang LP, Zou Q, Wu F, Zeng NK, Liu M (2020) Pseudohydnum brunneiceps (Auriculariales, Basidiomycota), a new species from Central China. Phytotaxa 441:87–94
Courtecuisse R, Lowy B (1990) Elements for a mycological inventory of the vicinity of ‘Saut Pararé’ (Arataye River) and ‘Nouragues Inselberg’ (French Guiana) III. Mycotaxon 39:329–344
Donk MA (1966) Check list of European hymenomycetous Heterobasidiae. Persoonia 4:145–335
Ellis JB, Everhart BM (1894) New species of fungi from various localities. Proc Acad Natl Sci Phila 46:322–384
Fries EM (1821) Systema mycologicum 1. Berling, Lund
Fries EM (1838) Epicrisis systematis mycologici. Typographia academica, Uppsala
Gardes M, Bruns TD (1993) ITS primers with enhanced specificity for basidiomycetes application to the identification of mycorrhizae and rusts. Mol Ecol 2:132–118. https://doi.org/10.1111/j.1365-294x.1993.tb00005.x
Holtermann C (1898) Mykologische Untersuchungen aus den Tropen. Bornträger, Berlin
Jacquin NJ (1778) Miscellanea austriaca ad botanicam, chemiam et historiam naturalem spectantia. Vol. 1. Kraus, Vienna
Kozlov AM, Darriba D, Flouri T, Morel B, Stamatakis A (2019) RAxML-NG: a fast, scalable, and user-friendly tool for maximum likelihood phylogenetic inference. Bioinformatics. https://doi.org/10.1093/bioinformatics/btz305
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol Biol Evol 35:1547–1549
Landvik S (1996) Neolecta, a fruit-body producing genus of the basal ascomycetes, as shown by SSU and LSU rDNA sequences. Mycol Res 100:199–202
Lowy B (1959) New and noteworthy Tremellales from Bolivia. Mycologia 51:840–850
Lowy B (1971) Tremellaceae. Flora Neotropica 6:1–153
Löytynoja A, Goldman N (2010) webPRANK: a phylogeny-aware multiple sequence aligner with interactive alignment browser. BMC Bioinformatics 11:579
Martin GW (1944) New and noteworthy tropical fungi. III Lloydia 7:67–80
Matheny PB, Liu YJ, Ammirati JF, Hall BD (2002) Using RPB1 sequences to improve phylogenetic inference among mushrooms (Inocybe, Agaricales). Am J Bot 89:688–698
Matheny PB, Wang Z, Binder M, Curtis JM, Lim YW, Nilsson RH, Hughes KW, Hofstetter V, Ammirati JF, Schoch CL, Langer E, Langer G, McLaughlin DJ, Wilson AW, Froslev T, Ge ZW, Kerrigan RW, Slot JC, Yang ZL, Baroni TJ, Fischer M, Hosaka K, Matsuura K, Seidl MT, Vauras J, Hibbett DS (2007) Contributions of RPB2 and TEF1 to the phylogeny of mushrooms and allies (Basidiomycota, Fungi). Mol Phyl Evol 43:430–451
Möller A (1895) Protobasidiomyceten. Botanische Mittheilungen Aus Den Tropen 8:1–180
Müller OF (1777) Flora Danica. Vol. 4. Copenhagen
Pilát A (1957) Übersicht der europäischen Auriculariales und Tremellales unter besonderer Berücksichtigung der tschechoslowakishen Arten. Acta Mus Nat Prag 13B:115–210
R Core Team (2018) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/. Accessed 1/11/2022
Rehner SA, Buckley E (2005) A Beauveria phylogeny inferred from nuclear ITS and EF1-alpha sequences: evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia 97:84–98
Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Hohna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61:539–542
RStudio Team (2022). RStudio: Integrated Development for R. RStudio, Inc., Boston. https://rstudio.com/. Accessed 1/11/2022
Scopoli JA (1772) Flora Carniolica. Vol. 2. Paul Krauss, Vienna
Spirin V, Malysheva V, Miettinen O, Vlasák J, Alvarenga RLM, Gibertoni TB, Ryvarden L, Larsson KH (2019) On Protomerulius and Heterochaetella (Auriculariales, Basidiomycota). Mycol Prog 18:1079–1099
Spirin V, Malysheva V, Alvarenga RLM, Kotiranta H, Larsson KH (2020) Studies in Basidiodendron eyrei and similar-looking taxa. Botany 98:623–638
Spirin V, Malysheva V, Schoutteten N, Viner I, Miettinen O, Nordén J, Ryvarden L, Kotiranta H, Verbeken A, Weiß M, Larsson KH (2021) Studies in the Basidiodendron caesiocinereum complex (Auriculariales, Basidiomycota). Mycol Prog 20:1275–1296
Spirin V, Malysheva V, Larsson KH (2018) On some forgotten species of Exidia and Myxarium (Auriculariales, Basidiomycota). Nordic J Bot 36(3):e01601:1–11
Swofford DL. 2002. PAUP – Phylogenetic Analysis Using Parsimony (and other methods). Version 4.0b10. Sinauer Associates; Sunderland, MA
Thiers B (2022) Index Herbariorum: a global directory of public herbaria and associated stuff [continuosly updated]. New York Botanical Garden’s Virtual Herbarium. http://sweetgum.nybg.org/ih. Accessed 1/11/2022
Vilgalys R, Hester M (1990) Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J Bacteriol 172:4238–4246
Warburg O (1899) Beiträge zur Kenntnis der Vegetation des Süd- und Ostasiatischen Monsungebietes. Monsunia 1:1–208
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA et al (eds) PCR protocols. Academic Press, San Diego (California), A guide to methods and applications, pp 315–322
Wojewoda W (1981) Mała flora grzybów. Tom 2. Krakow, Państwowe Wydawnictwo Naukowe Warsaw
Zhou HM, Liu HG, Gates GM, Wu F, Dai YC, Cooper JA (2022) Phylogeny and diversity of the genus Pseudohydnum (Auriculariales, Basidiomycota). J Fungi 8(658):1–15
Zhou HM, Bau T, Si J (2023) Morphological and phylogenetic evidence reveal three new Pseudohydnum (Auriculariales, Basidiomycota) species from North China. Front Cell Infect Microbiol 13(1139449):1–8
Acknowledgements
Kadri Pärtel (University of Tartu) sent us specimens from herbarium TAAM for a loan. Bart Buyck kindly arranged research visit of the author VS to herbarium PC. Andrej Piltvater provided us with valuable comments on Scopoli’s illustrations. Åsa Kruys (Uppsala University) sent us photographs of specimens from Fries’ herbarium. The authors are grateful to Olga Morozova for providing her collection of P. meridianum. CSC–IT Center for Science (Finland) provided computing resources for phylogenetic analyses.
Funding
Open Access funding provided by University of Helsinki including Helsinki University Central Hospital. The research was supported by the projects no. 315927 (Academy of Finland) and “A survey of Auriculariales and Sebacinales in Sweden” (SLU Artdatabanken) (the author VS), by the project no. 122011900033-4 (Komarov Botanical Institute RAS) (field work) and the agreement no. 075-15-2021-1056 (molecular study) (the authors VM and VD), by the research project J4-3098 “The unrevealed information on soil biodiversity in leached waters” and the Research Program in Forest Biology, Ecology, and Technology (P4-0107) of the Slovenian Research Agency (the author TG).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Viacheslav Spirin, Vera Malysheva, Ilya Viner, and Otto Miettinen. The first draft of the manuscript was written by Viacheslav Spirin, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Section Editor: Yu-Cheng Dai
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Spirin, V., Malysheva, V., Viner, I. et al. Taxonomy and multigene phylogeny of Pseudohydnum (Auriculariales, Basidiomycota). Mycol Progress 22, 40 (2023). https://doi.org/10.1007/s11557-023-01895-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11557-023-01895-4