Deposition of Chitosan on Plasma-Treated Polymers—A Review
Abstract
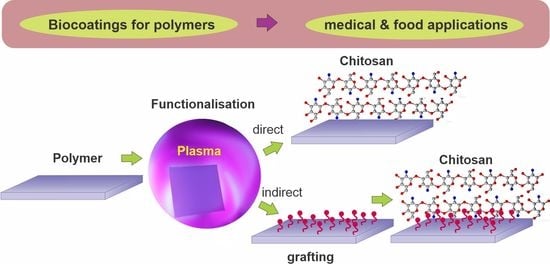
:1. Introduction
2. Surface Functionalization Using Plasma Methods
3. Overview of Plasma Methods for Chitosan Immobilization
4. Direct Deposition of Chitosan on Plasma-Treated Polymers
4.1. Examples of Low-Pressure Plasma Treatments for Direct Deposition of Chitosan
4.2. Examples of Atmospheric Pressure Plasma Treatments for Direct Deposition of Chitosan
5. Chitosan Deposition on Plasma-Treated Polymers with an Intermediate Layer
5.1. Examples of Low-Pressure Plasma Pretreatment for Indirect Deposition of Chitosan via an Intermediate Layer
5.2. Atmospheric Pressure Plasma Pretreatments for Indirect Deposition of Chitosan via an Intermediate Layer
6. Conclusions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Maitz, M.F. Applications of synthetic polymers in clinical medicine. Biosurface Biotribology 2015, 1, 161–176. [Google Scholar] [CrossRef] [Green Version]
- Neděla, O.; Slepička, P.; Švorčík, V. Surface modification of polymer substrates for biomedical applications. Materials 2017, 10, 1115. [Google Scholar] [CrossRef] [PubMed]
- Nemani, S.K.; Annavarapu, R.K.; Mohammadian, B.; Raiyan, A.; Heil, J.; Haque, M.A.; Abdelaal, A.; Sojoudi, H. Surface Modification of Polymers: Methods and Applications. Adv. Mater. Interfaces 2018, 5, 1801247. [Google Scholar] [CrossRef]
- Nathanael, A.J.; Oh, T.H. Biopolymer coatings for biomedical applications. Polymers 2020, 12, 3061. [Google Scholar] [CrossRef]
- Anjum, S.; Singh, S.; Benedicte, L.; Roger, P.; Panigrahi, M.; Gupta, B. Biomodification Strategies for the Development of Antimicrobial Urinary Catheters: Overview and Advances. Glob. Chall. 2018, 2, 1700068. [Google Scholar] [CrossRef] [Green Version]
- Klevens, R.M.; Edwards, J.R.; Richards, C.L., Jr.; Horan, T.C.; Gaynes, R.P.; Pollock, D.A.; Cardo, D.M. Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Public Health Rep. 2007, 122, 160–166. [Google Scholar] [CrossRef]
- Percival, S.L.; Suleman, L.; Vuotto, C.; Donelli, G. Healthcare-associated infections, medical devices and biofilms: Risk, tolerance and control. J. Med. Microbiol. 2015, 64, 323–334. [Google Scholar] [CrossRef] [Green Version]
- Dadi, N.C.T.; Radochová, B.; Vargová, J.; Bujdáková, H. Impact of Healthcare-Associated Infections Connected to Medical Devices—An Update. Microorganisms 2021, 9, 2332. [Google Scholar] [CrossRef]
- Leal, B.B.J.; Wakabayashi, N.; Oyama, K.; Kamiya, H.; Braghirolli, D.I.; Pranke, P. Vascular tissue engineering: Polymers and methodologies for small caliber vascular grafts. Front. Cardiovasc. Med. 2021, 7, 592361. [Google Scholar] [CrossRef]
- Bumgardner, J.D.; Wiser, R.; Gerard, P.D.; Bergin, P.; Chestnutt, B.; Marin, M.; Ramsey, V.; Elder, S.H.; Gilbert, J.A. Chitosan: Potential use as a bioactive coating for orthopaedic and craniofacial/dental implants. J. Biomater. Sci. Polym. Ed. 2003, 14, 423–438. [Google Scholar] [CrossRef]
- Teo, A.J.T.; Mishra, A.; Park, I.; Kim, Y.-J.; Park, W.-T.; Yoon, Y.-J. Polymeric biomaterials for medical implants and devices. ACS Biomater. Sci. Eng. 2016, 2, 454–472. [Google Scholar] [CrossRef]
- Amirtharaj Mosas, K.K.; Chandrasekar, A.R.; Dasan, A.; Pakseresht, A.; Galusek, D. Recent Advancements in Materials and Coatings for Biomedical Implants. Gels 2022, 8, 323. [Google Scholar] [CrossRef]
- Domingues, B.; Silva, J.M.; Aroso, I.M.; Lima, E.; Barros, A.A.; Reis, R.L. Coatings for Urinary Stents: Current State and Future Directions. In Urinary Stents: Current State and Future Perspectives; Soria, F., Rako, D., de Graaf, P., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 209–223. [Google Scholar]
- Singha, P.; Locklin, J.; Handa, H. A review of the recent advances in antimicrobial coatings for urinary catheters. Acta Biomaterialia 2017, 50, 20–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.; Kim, H.-h.; Yang, S.B.; Moon, J.-H.; Ahn, H.-W.; Hong, J. A Polysaccharide-Based Antibacterial Coating with Improved Durability for Clear Overlay Appliances. ACS Appl. Mater. Interfaces 2018, 10, 17714–17721. [Google Scholar] [CrossRef] [PubMed]
- Park, S.K.; Shin, J.H.; Jung, J.H.; Lee, D.Y.; Choi, D.Y.; Yoo, S.H. Polysaccharide-derivative coated intravascular catheters with superior multifunctional performance via simple and biocompatible method. Chem. Eng. J. 2022, 433, 134565. [Google Scholar] [CrossRef]
- Mendoza, G.; Regiel-Futyra, A.; Tamayo, A.; Monzon, M.; Irusta, S.; de Gregorio, M.A.; Kyzioł, A.; Arruebo, M. Chitosan-based coatings in the prevention of intravascular catheter-associated infections. J. Biomater. Appl. 2018, 32, 725–737. [Google Scholar] [CrossRef] [PubMed]
- Kou, S.; Peters, L.M.; Mucalo, M.R. Chitosan: A review of sources and preparation methods. Int. J. Biol. Macromol. 2021, 169, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Aljawish, A.; Chevalot, I.; Jasniewski, J.; Scher, J.; Muniglia, L. Enzymatic synthesis of chitosan derivatives and their potential applications. J. Mol. Catal. B Enzym. 2015, 112, 25–39. [Google Scholar] [CrossRef]
- Mutreja, R.; Thakur, A.; Goyal, A. Chapter 13—Chitin and chitosan: Current status and future opportunities. In Handbook of Chitin and Chitosan; Gopi, S., Thomas, S., Pius, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 401–417. [Google Scholar]
- Dima, J.B.; Sequeiros, C.; Zaritzky, N.E. In Chitosan from marine crustaceans: Production, characterization and applications. In Biological Activities and Application of Marine Polysaccharides; Intechopen: London, UK, 2017. [Google Scholar]
- Moratti, S.C.; Cabral, J.D. 2—Antibacterial properties of chitosan. In Chitosan Based Biomaterials Volume 1; Jennings, J.A., Bumgardner, J.D., Eds.; Woodhead Publishing: Sawston, UK, 2017; pp. 31–44. [Google Scholar]
- Ojeda-Hernández, D.D.; Canales-Aguirre, A.A.; Matias-Guiu, J.; Gomez-Pinedo, U.; Mateos-Díaz, J.C. Potential of chitosan and its derivatives for biomedical applications in the central nervous system. Front. Bioeng. Biotechnol. 2020, 8, 389. [Google Scholar] [CrossRef]
- Carlson, R.P.; Taffs, R.; Davison, W.M.; Stewart, P.S. Anti-biofilm properties of chitosan-coated surfaces. J. Biomater. Sci. Polym. 2008, 19, 1035–1046. [Google Scholar] [CrossRef]
- Gu, G.; Erişen, D.E.; Yang, K.; Zhang, B.; Shen, M.; Zou, J.; Qi, X.; Chen, S.; Xu, X. Antibacterial and anti-inflammatory activities of chitosan/copper complex coating on medical catheters: In vitro and in vivo. J. Biomed. Mater. Res.—B Appl. 2022, 110, 1899–1910. [Google Scholar] [CrossRef]
- Wang, B.-l.; Wang, J.-l.; Li, D.-d.; Ren, K.-f.; Ji, J. Chitosan/poly (vinyl pyrollidone) coatings improve the antibacterial properties of poly(ethylene terephthalate). Appl. Surf. Sci. 2012, 258, 7801–7808. [Google Scholar] [CrossRef]
- Teixeira-Santos, R.; Lima, M.; Gomes, L.C.; Mergulhão, F.J. Antimicrobial coatings based on chitosan to prevent implant-associated infections: A systematic review. iScience 2021, 24, 103480. [Google Scholar] [CrossRef] [PubMed]
- Sultankulov, B.; Berillo, D.; Sultankulova, K.; Tokay, T.; Saparov, A. Progress in the development of chitosan-based biomaterials for tissue engineering and regenerative medicine. Biomolecules 2019, 9, 470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, P.; Luo, Y.; Ke, C.; Qiu, H.; Wang, W.; Zhu, Y.; Hou, R.; Xu, L.; Wu, S. Chitosan-based functional materials for skin wound repair: Mechanisms and applications. Front. Bioeng. Biotechnol. 2021, 9, 650598. [Google Scholar] [CrossRef]
- Liu, H.; Wang, C.; Li, C.; Qin, Y.; Wang, Z.; Yang, F.; Li, Z.; Wang, J. A functional chitosan-based hydrogel as a wound dressing and drug delivery system in the treatment of wound healing. RSC Adv. 2018, 8, 7533–7549. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.M.; Shahruzzaman, M.; Biswas, S.; Nurus Sakib, M.; Rashid, T.U. Chitosan based bioactive materials in tissue engineering applications-A review. Bioact. Mater. 2020, 5, 164–183. [Google Scholar] [CrossRef]
- Loo, H.L.; Goh, B.H.; Lee, L.-H.; Chuah, L.H. Application of chitosan-based nanoparticles in skin wound healing. Asian J. Pharm. Sci. 2022, 17, 299–332. [Google Scholar] [CrossRef]
- Muxika, A.; Etxabide, A.; Uranga, J.; Guerrero, P.; de la Caba, K. Chitosan as a bioactive polymer: Processing, properties and applications. Int. J. Biol. Macromol. 2017, 105, 1358–1368. [Google Scholar] [CrossRef]
- Zargar, V.; Asghari, M.; Dashti, A. A Review on Chitin and Chitosan Polymers: Structure, Chemistry, Solubility, Derivatives, and Applications. ChemBioEng Rev. 2015, 2, 204–226. [Google Scholar] [CrossRef]
- Qin, Y.; Li, P. Antimicrobial chitosan conjugates: Current synthetic strategies and potential applications. Int. J. Mol. Sci. 2020, 21, 499. [Google Scholar] [CrossRef] [Green Version]
- Lunkov, A.P.; Ilyina, A.V.; Varlamov, V.P. Antioxidant, antimicrobial, and fungicidal properties of chitosan based films (Review). Appl. Biochem. Microbiol. 2018, 54, 449–458. [Google Scholar] [CrossRef]
- Sahariah, P.; Másson, M. Antimicrobial chitosan and chitosan derivatives: A review of the structure–activity relationship. Biomacromolecules 2017, 18, 3846–3868. [Google Scholar] [CrossRef]
- Rabea, E.I.; Badawy, M.E.T.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food. Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef]
- Fu, J.; Yang, F.; Guo, Z. The chitosan hydrogels: From structure to function. New J. Chem. 2018, 42, 17162–17180. [Google Scholar] [CrossRef]
- Wang, Q.Z.; Chen, X.G.; Liu, N.; Wang, S.X.; Liu, C.S.; Meng, X.H.; Liu, C.G. Protonation constants of chitosan with different molecular weight and degree of deacetylation. Carbohydr. Polym. 2006, 65, 194–201. [Google Scholar] [CrossRef]
- Arkhangelskiy, A.; Quaranta, A.; Motta, A.; Yang, Y.; Yadavalli, V.K.; Maniglio, D. Atmospheric plasma-assisted deposition and patterning of natural polymers. Adv. Mater. Interfaces 2022, 9, 2200454. [Google Scholar] [CrossRef]
- Bahrami, N.; Nouri Khorasani, S.; Mahdavi, H.; Ghiaci, M.; Mokhtari, R. Low-pressure plasma surface modification of polyurethane films with chitosan and collagen biomolecules. J. Appl. Polym. Sci. 2019, 136, 47567. [Google Scholar] [CrossRef]
- Karakurt, I.; Ozaltin, K.; Pištěková, H.; Vesela, D.; Michael-Lindhard, J.; Humpolícek, P.; Mozetič, M.; Lehocky, M. Effect of saccharides coating on antibacterial potential and drug loading and releasing capability of plasma treated polylactic acid films. Int. J. Mol. Sci. 2022, 23, 8821. [Google Scholar] [CrossRef] [PubMed]
- Sham, M.L.; Li, J.; Ma, P.C.; Kim, J.-K. Cleaning and functionalization of polymer surfaces and nanoscale carbon fillers by UV/Ozone treatment: A review. J. Compos. Mater. 2009, 43, 1537–1564. [Google Scholar] [CrossRef]
- Penn, L.S.; Wang, H. Chemical modification of polymer surfaces: A review. Polym. Adv. Technol. 1994, 5, 809–817. [Google Scholar] [CrossRef]
- Siau, S.; Vervaet, A.; Nalines, S.; Schacht, E.; Van Calster, A. Kinetic study of wet chemical treatments on the surface roughness of epoxy polymer layers for buildup layers: II. oxidative treatment of the surface. J. Electrochem. Soc. 2004, 151, C831. [Google Scholar] [CrossRef]
- Ghobeira, R.; Esbah Tabaei, P.S.; Morent, R.; De Geyter, N. Chemical characterization of plasma-activated polymeric surfaces via XPS analyses: A review. Surf. Interfaces 2022, 31, 102087. [Google Scholar] [CrossRef]
- Vesel, A.; Mozetic, M. New developments in surface functionalization of polymers using controlled plasma treatments. J. Phys. D Appl. Phys. 2017, 50, 293001. [Google Scholar] [CrossRef]
- Lari, E.S.; Askari, H.R.; Meftah, M.T.; Shariat, M. Calculation of electron density and temperature of plasmas by using new Stark broadening formula of helium lines. Phys. Plasmas 2019, 26, 023519. [Google Scholar] [CrossRef]
- Masruroh; Santjojo, D.J.D.H.; Abdurrouf; Abdillah, M.A.; Padaga, M.C.; Sakti, S.P. Effect of electron density and temperature in oxygen plasma treatment of polystyrene surface. IOP Conf. Ser. Mater. Sci. Eng. 2019, 515, 012061. [Google Scholar] [CrossRef]
- Urabe, K.; Shirai, N.; Tomita, K.; Akiyama, T.; Murakami, T. Diagnostics of atmospheric-pressure pulsed-dc discharge with metal and liquid anodes by multiple laser-aided methods. Plasma Sources Sci. Technol. 2016, 25, 045004. [Google Scholar] [CrossRef]
- Khorasani, M.T.; Mirzadeh, H. Effect of oxygen plasma treatment on surface charge and wettability of PVC blood bag—In vitro assay. Radiat. Phys. Chem. 2007, 76, 1011–1016. [Google Scholar] [CrossRef]
- Akhavan, B.; Jarvis, K.; Majewski, P. Development of negatively charged particulate surfaces through a dry plasma-assisted approach. RSC Adv. 2015, 5, 12910–12921. [Google Scholar] [CrossRef]
- Ershov, S.; Khelifa, F.; Druart, M.E.; Habibi, Y.; Olivier, M.G.; Snyders, R.; Dubois, P. Free radical-induced grafting from plasma polymers for the synthesis of thin barrier coatings. RSC Adv. 2015, 5, 14256–14265. [Google Scholar] [CrossRef]
- Dorai, R.; Kushner, M.J. A model for plasma modification of polypropylene using atmospheric pressure discharges. J. Phys. D Appl. Phys. 2003, 36, 666–685. [Google Scholar] [CrossRef] [Green Version]
- Zaplotnik, R.; Vesel, A. Effect of VUV radiation on surface modification of polystyrene exposed to atmospheric pressure plasma jet. Polymers 2020, 12, 1136. [Google Scholar] [CrossRef] [PubMed]
- Shin, G.H.; Lee, Y.H.; Lee, J.S.; Kim, Y.S.; Choi, W.S.; Park, H.J. Preparation of plastic and biopolymer multilayer films by plasma source ion implantation. J. Agric. Food Chem. 2002, 50, 4608–4614. [Google Scholar] [CrossRef]
- Jing, X.; Mi, H.-Y.; Peng, J.; Peng, X.-F.; Turng, L.-S. Electrospun aligned poly(propylene carbonate) microfibers with chitosan nanofibers as tissue engineering scaffolds. Carbohydr. Polym. 2015, 117, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Suganya, A.; Shanmugvelayutham, G.; Hidalgo-Carrillo, J. Plasma surface modified polystyrene and grafted with chitosan coating for improving the shelf lifetime of postharvest grapes. Plasma Chem. Plasma Process. 2018, 38, 1151–1168. [Google Scholar] [CrossRef]
- Glaser, T.K.; Plohl, O.; Vesel, A.; Ajdnik, U.; Ulrih, N.P.; Hrnčič, M.K.; Bren, U.; Fras Zemljič, L. Functionalization of polyethylene (PE) and polypropylene (PP) material using chitosan nanoparticles with incorporated resveratrol as potential active packaging. Materials 2019, 12, 2118. [Google Scholar] [CrossRef] [Green Version]
- Komoto, D.; Ikeda, R.; Furuike, T.; Tamura, H. Preparation of chitosan-coated poly(L-lactic acid) fibers for suture threads. Fibers 2018, 6, 84. [Google Scholar] [CrossRef] [Green Version]
- Nawalakhe, R.; Shi, Q.; Vitchuli, N.; Noar, J.; Caldwell, J.M.; Breidt, F.; Bourham, M.A.; Zhang, X.; McCord, M.G. Novel atmospheric plasma enhanced chitosan nanofiber/gauze composite wound dressings. J. Appl. Polym. Sci. 2013, 129, 916–923. [Google Scholar] [CrossRef]
- Lei, J.; Yang, L.; Zhan, Y.; Wang, Y.; Ye, T.; Li, Y.; Deng, H.; Li, B. Plasma treated polyethylene terephthalate/polypropylene films assembled with chitosan and various preservatives for antimicrobial food packaging. Colloid. Surf. B 2014, 114, 60–66. [Google Scholar] [CrossRef]
- Ren, Y.; Ding, Z.; Wang, C.; Zang, C.; Zhang, Y.; Xu, L. Influence of DBD plasma pretreatment on the deposition of chitosan onto UHMWPE fiber surfaces for improvement of adhesion and dyeing properties. Appl. Surf. Sci. 2017, 396, 1571–1579. [Google Scholar] [CrossRef]
- Carette, X.; Mincheva, R.; Herbin, M.; Noirfalise, X.; Nguyen, T.C.; Leclere, P.; Godfroid, T.; Kerdjoudj, H.; Jolois, O.; Boudhifa, M.; et al. Atmospheric plasma: A simple way of improving the interface between natural polysaccharides and polyesters. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1056, 012005. [Google Scholar] [CrossRef]
- Tkavc, T.; Petrinič, I.; Luxbacher, T.; Vesel, A.; Ristić, T.; Zemljič, L.F. Influence of O2 and CO2 plasma treatment on the deposition of chitosan onto polyethylene terephthalate (PET) surfaces. Int. J. Adhes. Adhes. 2014, 48, 168–176. [Google Scholar] [CrossRef]
- Pâslaru, E.; Fras Zemljic, L.; Bračič, M.; Vesel, A.; Petrinić, I.; Vasile, C. Stability of a chitosan layer deposited onto a polyethylene surface. J. Appl. Polym. Sci. 2013, 130, 2444–2457. [Google Scholar] [CrossRef]
- Terpiłowski, K.; Wiącek, A.E.; Jurak, M. Influence of nitrogen plasma treatment on the wettability of polyetheretherketone and deposited chitosan layers. Adv. Polym. Technol. 2018, 37, 1557–1569. [Google Scholar] [CrossRef] [Green Version]
- Demina, T.S.; Piskarev, M.S.; Romanova, O.A.; Gatin, A.K.; Senatulin, B.R.; Skryleva, E.A.; Zharikova, T.M.; Gilman, A.B.; Kuznetsov, A.A.; Akopova, T.A.; et al. Plasma treatment of poly(ethylene terephthalate) films and chitosan deposition: DC- vs. AC-discharge. Materials 2020, 13, 508. [Google Scholar] [CrossRef] [Green Version]
- Tyan, Y.C.; Liao, J.D.; Lin, S.P. Surface properties and in vitro analyses of immobilized chitosan onto polypropylene nonwoven fabric surface using antenna-coupling microwave plasma. J. Mater. Sci. Mater. Med. 2003, 14, 775–781. [Google Scholar] [CrossRef]
- Lin, W.-C.; Tseng, C.-H.; Yang, M.-C. In-vitro hemocompatibility evaluation of a thermoplastic polyurethane membrane with surface-immobilized water-soluble chitosan and heparin. Macromol. Biosci. 2005, 5, 1013–1021. [Google Scholar] [CrossRef]
- Zhu, A.; Chen, T. Blood compatibility of surface-engineered poly(ethylene terephthalate) via o-carboxymethylchitosan. Colloid. Surf. B 2006, 50, 120–125. [Google Scholar]
- Pandiyaraj, K.N.; Ferraria, A.M.; Rego, A.M.B.d.; Deshmukh, R.R.; Su, P.-G.; Halleluyah, J.M.; Halim, A.S. Low-pressure plasma enhanced immobilization of chitosan on low-density polyethylene for bio-medical applications. Appl. Surf. Sci. 2015, 328, 1–12. [Google Scholar] [CrossRef]
- Navaneetha Pandiyaraj, K.; Ram Kumar, M.C.; Arun Kumar, A.; Padmanabhan, P.V.A.; Deshmukh, R.R.; Bah, M.; Ismat Shah, S.; Su, P.-G.; Halleluyah, M.; Halim, A.S. Tailoring the surface properties of polypropylene films through cold atmospheric pressure plasma (CAPP) assisted polymerization and immobilization of biomolecules for enhancement of anti-coagulation activity. Appl. Surf. Sci. 2016, 370, 545–556. [Google Scholar] [CrossRef]
- Tardajos, M.G.; Cama, G.; Dash, M.; Misseeuw, L.; Gheysens, T.; Gorzelanny, C.; Coenye, T.; Dubruel, P. Chitosan functionalized poly-ε-caprolactone electrospun fibers and 3D printed scaffolds as antibacterial materials for tissue engineering applications. Carbohydr. Polym. 2018, 191, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Vaz, J.M.; Taketa, T.B.; Hernandez-Montelongo, J.; Chevallier, P.; Cotta, M.A.; Mantovani, D.; Beppu, M.M. Antibacterial properties of chitosan-based coatings are affected by spacer-length and molecular weight. Appl. Surf. Sci. 2018, 445, 478–487. [Google Scholar] [CrossRef]
- Pandiyaraj, K.N.; Ramkumar, M.C.; Arun Kumar, A.; Padmanabhan, P.V.A.; Pichumani, M.; Bendavid, A.; Cools, P.; De Geyter, N.; Morent, R.; Kumar, V.; et al. Evaluation of surface properties of low density polyethylene (LDPE) films tailored by atmospheric pressure non-thermal plasma (APNTP) assisted co-polymerization and immobilization of chitosan for improvement of antifouling properties. Mat. Sci. Eng. C 2019, 94, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Popelka, A.; Novák, I.; Lehocký, M.; Junkar, I.; Mozetič, M.; Kleinová, A.; Janigová, I.; Šlouf, M.; Bílek, F.; Chodák, I. A new route for chitosan immobilization onto polyethylene surface. Carbohydr. Polym. 2012, 90, 1501–1508. [Google Scholar] [CrossRef]
- Asadinezhad, A.; Novák, I.; Lehocký, M.; Bílek, F.; Vesel, A.; Junkar, I.; Sáha, P.; Popelka, A. Polysaccharides coatings on medical-grade PVC: A probe into surface characteristics and the extent of bacterial adhesion. Molecules 2010, 15, 1007–1027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vesel, A.; Recek, N.; Zaplotnik, R.; Kurinčič, A.; Kuzmič, K.; Zemljič, L.F. A method for the immobilization of chitosan onto urinary catheters. Int. J. Mol. Sci. 2022, 23, 15075. [Google Scholar] [CrossRef]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for biomedical applications: Their characteristics and the mechanisms behind them. Gels 2017, 3, 6. [Google Scholar] [CrossRef] [Green Version]
- Pellá, M.C.G.; Lima-Tenório, M.K.; Tenório-Neto, E.T.; Guilherme, M.R.; Muniz, E.C.; Rubira, A.F. Chitosan-based hydrogels: From preparation to biomedical applications. Carbohydr. Polym. 2018, 196, 233–245. [Google Scholar] [CrossRef]
- Carette, X.; Mincheva, R.; Herbin, M.; Cabecas Segura, P.; Wattiez, R.; Noirfalise, X.; Thai, C.; Leclere, P.; Godfroid, T.; Boudifa, M.; et al. Microwave atmospheric plasma: A versatile and fast way to confer antimicrobial activity toward direct chitosan immobilization onto poly(lactic acid) substrate. ACS Appl. Bio Mater. 2021, 4, 7445–7455. [Google Scholar] [CrossRef]
- Li, Y.; Ren, J.; Wang, B.; Lu, W.; Wang, H.; Hou, W. Development of biobased multilayer films with improved compatibility between polylactic acid-chitosan as a function of transition coating of SiOx. Int. J. Biol. Macromol. 2020, 165, 1258–1263. [Google Scholar] [CrossRef] [PubMed]
- Budnyak, T.M.; Vlasova, N.N.; Golovkova, L.P.; Markitan, O.; Baryshnikov, G.; Ågren, H.; Slabon, A. Nucleotide interaction with a chitosan layer on a silica surface: Establishing the mechanism at the molecular level. Langmuir 2021, 37, 1511–1520. [Google Scholar] [CrossRef] [PubMed]
- Nicolle, L.; Journot, C.M.A.; Gerber-Lemaire, S. Chitosan functionalization: Covalent and non-covalent interactions and their characterization. Polymers 2021, 13, 4118. [Google Scholar] [CrossRef] [PubMed]
- Uthaiwat, P.; Priprem, A.; Puthongking, P.; Daduang, J.; Nukulkit, C.; Chio-Srichan, S.; Boonsiri, P.; Thapphasaraphong, S. Characteristic evaluation of gel formulation containing niosomes of melatonin or Its derivative and mucoadhesive properties using ATR-FTIR spectroscopy. Polymers 2021, 13, 1142. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Wang, Z.; Rao, W.; Cao, L.; Zhang, D. Molecular interaction analysis between collagen and chitosan blend film based on infrared spectroscopy. Trans. Chin. Soc. Agric. Eng. 2018, 34, 285–291. [Google Scholar]
- Bruce, R.L.; Weilnboeck, F.; Lin, T.; Phaneuf, R.J.; Oehrlein, G.S.; Long, B.K.; G.Willson, C.; Vegh, J.J.; Nest, D.; Graves, D.B. Relationship between nanoscale roughness and ion-damaged layer in argon plasma exposed polystyrene films. J. Appl. Phys. 2010, 107, 084310. [Google Scholar] [CrossRef]
- Mozetič, M. Plasma-stimulated super-hydrophilic surface finish of polymers. Polymers 2020, 12, 2498. [Google Scholar] [CrossRef]
- Husain, E.; Nema, R.S. Analysis of Paschen curves for air, N2 and SF6 using the Townsend breakdown equation. IEEE Trans. Dielectr. Electr. Insul. 1982, EI-17, 350–353. [Google Scholar] [CrossRef]
- Günther, R.; Caseri, W.R.; Brändli, C. Direct bonding and de-bonding on demand of polystyrene and polyamide surfaces, treated with oxygen plasma. J. Appl. Polym. Sci. 2022, 139, 51753. [Google Scholar] [CrossRef]
- Vesel, A.; Zaplotnik, R.; Mozetič, M.; Primc, G. Surface modification of PS polymer by oxygen-atom treatment from remote plasma: Initial kinetics of functional groups formation. Appl. Surf. Sci. 2021, 561, 150058. [Google Scholar] [CrossRef]
- Surya Arinda, P.; Joseph Djoko Herry Santjojo, D.; Masruroh, M.; Purnomo Sakti, S. Stability of polystyrene film surface wettability modified using oxygen plasma. Mater. Today Proc. 2019, 13, 24–29. [Google Scholar] [CrossRef]
- Beaulieu, I.; Geissler, M.; Mauzeroll, J. Oxygen plasma treatment of polystyrene and Zeonor: Substrates for adhesion of patterned cells. Langmuir 2009, 25, 7169–7176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golda, J.; Biskup, B.; Layes, V.; Winzer, T.; Benedikt, J. Vacuum ultraviolet spectroscopy of cold atmospheric pressure plasma jets. Plasma Process. Polym. 2020, 17, 1900216. [Google Scholar] [CrossRef] [Green Version]
- Stevie, F.A.; Donley, C.L. Introduction to x-ray photoelectron spectroscopy. J. Vac. Sci. Technol. A 2020, 38, 063204. [Google Scholar] [CrossRef]
- Booth, J.-P.; Mozetič, M.; Nikiforov, A.; Oehr, C. Foundations of plasma surface functionalization of polymers for industrial and biological applications. Plasma Sources Sci. Technol. 2022, 31, 103001. [Google Scholar] [CrossRef]
- Stoleru, E.; Vasile, C.; Irimia, A.; Brebu, M. Towards a bioactive food packaging: Poly(Lactic Acid) surface functionalized by chitosan coating embedding clove and argan oils. Molecules 2021, 26, 4500. [Google Scholar] [CrossRef]
- Vesel, A.; Zaplotnik, R.; Primc, G.; Mozetič, M.; Katan, T.; Kargl, R.; Mohan, T.; Kleinschek, K.S. Rapid functionalization of polytetrafluorethylene (PTFE) surfaces with nitrogen functional groups. Polymers 2021, 13, 4301. [Google Scholar] [CrossRef]
- Kafedjiiski, K.; Föger, F.; Werle, M.; Bernkop-Schnürch, A. Synthesis and in vitro evaluation of a novel chitosan–glutathione conjugate. Pharm. Res. 2005, 22, 1480–1488. [Google Scholar] [CrossRef]
















| Reference and Year | Substrate Material | Type of Discharge | Type of Gas | Gas Pressure | Treatment Time | Chitosan Concentration and Incubation Time | WCA of Untreated Substrate | WCA of Treated Substrate | WCA of Chitosan—Coated Samples | Thickness of Chitosan |
|---|---|---|---|---|---|---|---|---|---|---|
| [58] 2002 | Linear LDPE | Ion implantation, RF + pulsed DC | O2 | 0.17 Pa | 60 s | 3% (w/v); deposited and dried | 86° | 6° | N/A | 50 µm |
| [59] 2015 | PPC microfibers | RF CCP | O2 | 0.04 Pa | 36 s | 0.03–0.07 mg/mL; 2 min | 122° | 92° | 53° | N/A |
| [60] 2018 | PS | DC | Ar, O2, air | 2 Pa | 5–30 min | 1% (w/v); 5 min | 78° | ~40° | 80–92° | N/A |
| [61] 2019 | PE, PP | MW-surfatron | O2 | 50 Pa | 60 s | 2% (w/v); N/A | 108, 109° | 30, 37° | 42, 45° | ~1 mm (estimated from its mass 0.1 g/cm2) |
| [62] 2018 | PLA fibers | RF | O2 | N/A | 30–1800 s | 1% (w/v); 1 min | N/A | N/A | N/A | 30–100 nm |
| [63] 2013 | Cotton gauze | DBD | He | 105 Pa (1 bar) | N/A | 2–7%, electrospinning, 2 h | N/A | N/A | N/A | Nanofibers with a diameter of 250 nm |
| [64] 2014 | PP | Atmospheric DBD | Air | 105 Pa (1 bar) | 180 s | 2% (w/v); 1 min | 92° | 68° | 39° | N/A |
| [65] 2017 | PE fibers | DBD | Ar/O2 (90:10) | 105 Pa (1 bar) | 40–140 s | 0.7% (w/v); N/A | 114° | N/A | 81–95° | N/A, very thin |
| [66,84] 2021 | PLA | MW | Ar | 105 Pa (1 bar) | N/A | 1% (w/v); 10 s | 85° | 55° | N/A | N/A |
| [67] 2014 | PET | RF ICP | O2 or CO2 | 75 Pa | 30 s | 1.5% (w/v); 72 h | 92° | 28° or 35° | N/A | N/A |
| [68] 2013 | PE | Corona | Air | 105 Pa (1 bar) | N/A | 1 wt %, N/A | N/A | N/A | N/A | 30 μm |
| [69] 2018 | PEEK | RF CCP | Air followed by N2 | 20 Pa | 1 min + 1 min | 0.1 mg/mL, deposited and dried | 67° | 5° | 52° | N/A |
| [70] 2020 | PET | DC or AC | Air | 20 Pa | 10–60 s | 1% (w/v); 2 h | 80° | 10° or 17° | ~50° | N/A |
| [42] 2021 | PET, PDMS | Torch | Chitosan | N/A | 1 run | N/A | N/A | N/A | N/A | N/A |
| [81] 2022 | Meliflex XP polyolefin | RF | H2 followed by O2 | 20 Pa 45 Pa | 30 s 2 s | 2, 2.5% (w/v); 20 min | 96–103° | 37–46° | 82–100° | N/A |
| Reference | Substrate Material | Type of Discharge | Type of Gas | Gas Pressure | Treatment Time | Intermediate Step (Grafting and Coupling Agent) | Chitosan Concentration and Incubation Time | WCA of Untreated Substrate | WCA of Treated Substrate | WCA of Intermediate Layer | WCA of Chitosan-Coated Samples | Thickness of Chitosan |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [71] 2003 | Nonwoven PE | MW | O2 | 33 Pa | 10 s | AA and EDC | 0.3% (w/v), 24 h | N/A | Small | N/A | N/A | N/A |
| [72] 2005 | PU membrane | RF CCP | O2 | Low | 20–150 s | AA and EDC/NHS | 0.25 mg/mL; 24 h | 68° | N/A | N/A | 50° | N/A |
| [73] 2006 | PET | RIE, RF CCP | Ar | Low | 180 s | AA and DECH | 0.3% (w/v), 24 h | 75° | N/A | 34° | 21° | N/A |
| [80] 2010 | PVC | DCSBD | Air | 105 Pa (1 bar) | 15 s | AA and EDAC | 1% (w/v), 20 min | 86° | 65° | 46° | 63° | N/A |
| [79] 2012 | LDPE | DCSBD | Air | 105 Pa (1 bar) | 15 s | AA and EDAC | 1% (w/v), 24 h | 99° | 77° | 67° | 69° | N/A |
| [68] 2013 | PE | Corona | Air | 105 Pa (1 bar) | N/A | No grafting; EDC/NHS | 1 wt %, N/A | N/A | N/A | N/A | N/A | 25 μm |
| [74] 2015 | LDPE | DC, magnetized | Ar | 20 Pa | 300 s | AA, PEG, no agent | 1% (w/v), 1 min | 95° | 57° | 22° | 16° | N/A, very thin |
| [75] 2016 | PP | DBD | Ar | 105 Pa (1 bar) | 60 s | AA, PEG, and EDC | 1% (w/v), 30 min | 92° | 65° | 20° | 16° | N/A, very thin |
| [76] 2018 | PCL fibers | RF CCP | Ar | 80 Pa | 30 s | NHSMA, no agent | 0.5–1% (w/v) Overnight | N/A | N/A | N/A | N/A | N/A, very thin |
| [77] 2018 | PTFE | DBD | N2/H2 (95:5) | 105 Pa (1 bar) | 45 s | GA, PEGb, or PA and EDAC | 2% (w/v), 3 h | 100° | N/A | N/A | 50–60° | N/A |
| [78] 2019 | LDPE | DBD | Ar +PEG and AA vapour | 105 Pa (1 bar) | 60 s + 5 min | AA, PEG | 1% (w/v), 30 min | 95° | 13° | 13° | 0° | N/A, thin |
| [43] 2019 | PU | RF CCP | N2 | 20 Pa | 120 s | AA and EDC/NHS | 0.1%, 8 h | 80° | 20° | 45° | 85° | N/A |
| [85] 2020 | PLA | PECVD | HDMSO/O2 | N/A | 30, 60 s | SiOx | 1% (w/v) | 51° | N/A | 34° | N/A | 40 μm |
| [42] 2021 | PLA | RF | N2 | 40 Pa | N/A | EDC | 1.3% (w/v) | 89.7° | N/A | N/A | 73.5° | N/A |
| [44] 2022 | PLA | RF CCP | Air | 60 Pa | 60 s | AA and EDC and NHS | N/A | 82° | 46° | N/A | 65° | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vesel, A. Deposition of Chitosan on Plasma-Treated Polymers—A Review. Polymers 2023, 15, 1109. https://doi.org/10.3390/polym15051109
Vesel A. Deposition of Chitosan on Plasma-Treated Polymers—A Review. Polymers. 2023; 15(5):1109. https://doi.org/10.3390/polym15051109
Chicago/Turabian StyleVesel, Alenka. 2023. "Deposition of Chitosan on Plasma-Treated Polymers—A Review" Polymers 15, no. 5: 1109. https://doi.org/10.3390/polym15051109