Capital-Income Breeding in Male Ungulates: Causes and Consequences of Strategy Differences Among Species
- 1Department of Veterinary Medicine, University of Sassari, Sassari, Italy
- 2Agriculture and Wildlife Service, Piacenza, Italy
- 3Environmental Protection College, Velenje, Slovenia
- 4Slovenian Forestry Institute, Ljubljana, Slovenia
- 5Department of Biosciences, Durham University, Durham, United Kingdom
The capital and income breeding concept links energy resources used during reproduction to the timing of their acquisition. During reproduction, capital breeders rely on resources gained previously and accumulated for reproductive investment. By contrast, income breeders use mainly resources collected during the period of reproductive activity. Most commonly, this concept is applied to females; relatively few studies have considered males. Moreover, there has been little attention to the link between the capital-income divide and other aspects of mating strategy. We studied adult males of three wild ungulates with different levels of polygyny. A large dataset (4,264 red deer, 53,619 roe deer, and 13,537 Alpine chamois, respectively) was obtained during 2007–2017 in the whole territory of Slovenia and in the Trento province, Italy. During the rut, body mass loss of males in highly polygynous species was more than twice that of weakly polygynous species: on average, red deer stags lost 19.5%; chamois bucks 16.0%; and roe deer bucks 7.5% of their body mass. This indicates potential for a hitherto unrecognized link between the degree of intrasexual competition and the degree of capital mating. The variability in body mass at the end of the rut was clearly reduced in both highly polygynous species (from 15.1 to 9.4% in red deer, and from 12.5 to 10.5% in chamois), but did not change in roe deer. Finally, roe deer bucks had recovered body mass to that of the pre-rut period by just 2 months after the rut, while red deer stags did not manage to compensate the loss of weight until the end of the year. We suggest that, at least in ungulates, there is a link between the degree of polygyny and that of capital breeding. Males of capital and income breeders underwent body mass changes resulting from different reproductive investment during the rut. Capital breeders lost considerably more weight, and invested a variable amount of energy among individuals or among years, possibly to cope with different environmental or body conditions. In so doing, they ended the rut with poorer but more even condition among individuals.
Introduction
Capital and income breeding concepts were developed to help differentiate broad strategies for “financing” the costs of reproduction in animal species: “capital breeding” describes the situation in which reproduction is financed using stored capital; by contrast, “income breeding” refers to the use of concurrent intake to pay for a reproductive attempt (Drent and Daan, 1980; Jönsson, 1997). Recently, these strategies have been recognized to lie toward either end of a continuum of capital-to-income approaches to reproduction, and that placement along that continuum can vary among age classes of the same sex (Houston et al., 2007; Jaatinen et al., 2016; Williams et al., 2017).
Initially, most of the effort to understand both capital and income breeding and the differences between them was devoted to studying females at different phases of breeding from conception to egg laying, incubation and offspring-rearing in birds, and from conception to embryonic development, birth and lactation in mammals (Drent and Daan, 1980; Andersen et al., 2000; Boyd, 2000; Connan et al., 2019). Far fewer studies, however, considered males (but see Soulsbury, 2019), for which the contribution to reproduction is usually lower than for females. Despite this, reproduction may be demanding for males of some species, especially those – like ungulates – characterized by high polygyny (Mysterud et al., 2004). In male ungulates, which show no parental care, the relevant time period of reproductive investment is obviously the rut, because once conception is ensured, males have no further involvement in reproduction. In this context, the obvious metric to estimate the investment of rutting males is their loss of weight during the rut (Mysterud et al., 2005).
In ungulates, adult males show the highest loss of body mass during the rut, whereas younger males that have yet to reach social maturity show more limited loss, if any (for Alpine chamois Rupicapra rupicapra: Mason et al., 2011; for moose Alces alces: Mysterud et al., 2005; for American bison Bison bison: Komers et al., 1994; for red deer Cervus elaphus: Bobek et al., 1990; Milner et al., 2002; Yoccoz et al., 2002; for fallow deer Dama dama: McElligott et al., 2003; for reindeer Rangifer tarandus: Kojola, 1991; Mysterud et al., 2003). Moreover, adult males are the age class in which hypophagia is more evident (in moose: Miquelle, 1990; fallow deer: Apollonio and Di Vittorio, 2004; reindeer: Barboza et al., 2004; Alpine ibex: Brivio et al., 2010; American bison: Bergman et al., 2001; bighorn sheep Ovis canadensis: Pelletier et al., 2005; Alpine chamois: Willisch and Ingold, 2007).
The hypothesis that gradation from income to capital breeding, reflecting the importance of foraging strategies for reproduction in adults, has gained acceptance. Moreover, the degree of capital (vs. income) breeding is correlated with various ecological, morphological, and physiological traits (Stephens et al., 2009 for review). As yet, however, mating systems – and, more specifically, the degree of polygyny associated with different systems – have not been recognized as potential drivers of the adoption of capital or income breeding strategies. This is the case, despite the likelihood that mating systems could play a strong role, especially for males in systems with a defined breeding period and no paternal care. Socially adult males are ideal candidates for testing this hypothesis, because they are fully involved in bearing the costs of reproduction during rut (Mysterud et al., 2004).
In mammalian polygynous mating systems, male mating success is strongly linked to the level of monopolization of females and is related to the amount of energy devoted to intra-male competition (Clutton-Brock, 1989). In those systems, the major cost of reproduction for males must be associated with mating and the acquisition of mates. Moreover, given that high polygyny is associated with high competition, the intensity of competition is likely to favor the suppression of feeding (i.e., capital breeding) in highly polygynous males. By contrast, if there is low polygyny and low competition, males can continue to feed (i.e., income breeding). Alternatively, the low potential gains in systems characterized by low levels of polygyny will reduce the incentive to cease eating (and vice versa for high polygyny).
Our reasoning suggests that the degree of polygyny associated with different mating systems could be a powerful predictor of the adoption of either capital or income breeding. To test such hypotheses, a group of species that vary in their degree of polygyny, and for which reproductive investment can be determined whilst accounting for competing predictors of apparent investment must be identified. European ungulates present a useful opportunity for this analysis, exhibiting wide variability in polygyny with, as a result of the climatic range across which they occur, the simultaneous opportunity to compare effects of the environment on patterns of mass gain and loss.
Different levels of polygyny are shown by different standardized variances in male lifetime reproductive success or by different degrees of sexual dimorphism. At present, reliable data on variance in male lifetime reproductive success in mammals are limited (in red deer: Pemberton et al., 1992; Marshall et al., 1998; in bighorn sheep: Coltman et al., 2002; in Soay sheep Ovis aries: Coltman et al., 1999; in roe deer Capreolus capreolus: Vanpé et al., 2009). Degree of polygyny also can be shown by a relationship with sexual size dimorphism (SSD), especially in those polygynous species in which fighting between males involves wrestling or ramming. This outcome occurs because species with high SSD tend to have highly polygynous mating systems (for a review, see Alexander et al., 1979; and for ungulates, see: Loison et al., 1999; Vanpé et al., 2008). Associations between the degree of capital breeding and the phenotypic difference between males and females (i.e., secondary sex characteristics, body size, physical strength and morphology, ornamentation, and other bodily traits) can therefore be evaluated.
We analyzed a substantial sample of >65,000 individual males, belonging to three common European ungulate species: red deer, roe deer, and Alpine chamois. Males of those species differ in size, with undressed body weights (mean ± ES) of adult males from our sample of 163.0 ± 1.5 kg for red deer, 27.2 ± 0.1 kg for Alpine chamois, and 18.7 ± 0.03 kg for roe deer. More importantly for our study, clear differences exist in the mating systems adopted by the three species. Specifically, in the social red deer and Alpine chamois, males monopolize access to female groups, whilst in the more solitary roe deer, males guard only one female at any time. Red deer adopt a mating system in which a harem or territory-holding male monopolizes from 1 to 22 females (mean 3.6 per day) (Clutton-Brock et al., 1982) and territorial males up to a mean of 2.8 females per day (Carranza et al., 1990). In Alpine chamois, territorial and non-territorial males vie to monopolize groups of three to five females per hour during the rut (Corlatti et al., 2013b; Chirichella et al., unpublished). In contrast, roe deer bucks are long-term territory holders, but females are solitary, so territorial males have access only to one female at any time, i.e., only to the females either present in their territory (Kurt, 1991; Lieberg et al., 1998) or females that visit them during reproductive excursions (Debeffe et al., 2014).
Our objectives were as follows: (i) to test if species with a higher degree of polygyny are characterized by a capital breeding strategy, whilst the less polygynous roe deer is an income breeder; (ii) to evaluate the seasonal dynamics of weight loss in males of all three studied species, examining variance of body mass before and after the rut; and (iii) to assess the roles of location and year of sampling in determining variation in apparent investment among adult males.
Materials and Methods
Study Areas and Sampling
Data were collected in Italy, in the Central-Eastern Italian Alps, across a 6,207 km2 area in the Trento province (46°04’N, 11°07’E), and throughout Slovenia, across a 20,273 km2 area (46°03’N, 14°30’E). All individuals used in the study were legally hunted during the regular hunting activity prescribed by the state of Slovenia and Italy within yearly hunting-management plans. We used only data on dead individuals; therefore, no animal was shot or killed by any other means for the purposes of the research.
Slovenia is located in the transition zone between four macrogeographical units (Sub-Mediterranean, Alpine and Pre-Alpine, Karst-Dinaric, Pre-Pannonian), therefore it has a mixture of temperate continental, mountain (moderate Alpine), and Sub-Mediterranean climates (Ogrin, 1996; Perko, 1998; Lovenčak, 1999). Considering presence-abundance and ecological differentiation among populations of studied species (for red deer: Hafner, 2008; roe deer: Flajšman, 2017; chamois: Bužan et al., 2013) we located each individual in one of those macrogeographical units. In the Sub-Mediterranean region, vegetation is dominated by deciduous tree and shrub species. Mean temperature of the coldest month (mean January temperature) is above 0°C, and mean temperature of the warmest month (mean July temperature) is above 20°C. Mean annual precipitation is between 1,000 and 1,700 mm. The Alpine and Pre-Alpine region covers high mountains, lower hills and plains. Up to the tree line, there is mostly mixed forest. Mean temperature of the coldest month is below -3°C, whereas mean temperature of the warmest month depends on the altitude: in lower altitudes, it is above 10°C, and in higher altitudes [>1,500 m above sea level (asl)] below 10°C. Mean annual precipitation is between 1,100 and 3,000 mm. The Karst-Dinaric region consists of Dinaric plateaus and valley systems and is mainly covered by large complexes of beach-fir forest. The Pre-Pannonian region covers hilly and lowland area that extends toward the Pannonian plain. This region is mostly covered with cultivated land and broadleaf forests. In both Karst-Dinaric and Pre-Pannonian regions, there is a continental climate. Mean temperature of the coldest month is between 0 and -3°C, and the mean temperature of the warmest month is between 15 and 20°C. Mean annual precipitation in the Karst-Dinaric region is between 1,300 and 2,800 mm, and in the Pre-Pannonian region between 800 and 1,000 mm.
Variability in the climate of Trento province is similar, because of its geographical position and rich variety of landscapes. The climate in this area can be defined as a transition between the semi-continental and the Alpine climate. Temperature and rain are influenced by the Mediterranean climate in the southern part, while the northern part has a more continental climate. Average winter temperatures are between -5 and -10°C in January, and average summer temperatures are 20–25°C or more. Average annual rainfall is 815 mm. Nonetheless, rainfall varies according to the altitude and exposition of the relief. In general, the greatest rainfall falls on the highest peaks and in the southern and western sectors, where the western and southern winds that accompany the passage of the Atlantic disturbances bring humidity: here, rainfall amounts to 1,200–1,400 mm/year. The peaks of rainfall generally occur during autumn and spring whereas in winter, snowfall prevails. A large part of the Trento province is at relatively high average altitude (about 77% above 1,000 m asl; slightly <20% above 2,000 m asl); snow cover has an extremely irregular pattern (with some very snowy and other very dry years), and has exhibited a decrease in snow depth from the late 1980s. This decrease is more evident in the pre-Alpine areas and can have high variation based on the exposure (data from Forecasts and Organization Office – Civil Protection Infrastructures Department of the Trento province)1.
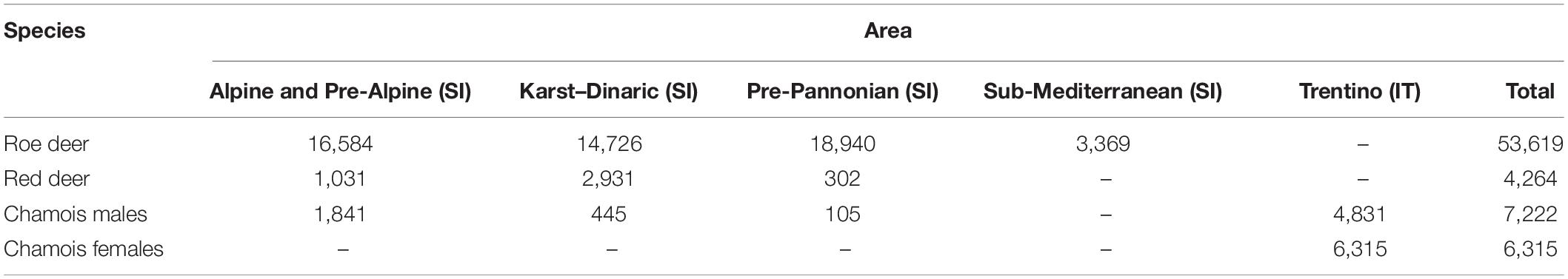
Around 58% of Slovenia is covered by forest (1.2 million ha), and its elevation ranges from sea level to 2,864 m asl. Slovenia is divided into 15 hunting management districts (HMD), subdivided into 411 hunting grounds managed by hunting clubs, and 12 hunting grounds with special purposes managed by the public organizations (i.e., Slovenia Forest Service, State Protocol, and the Triglav National Park). Roe deer are abundant throughout Slovenia, while abundances of red deer and Alpine chamois in Sub-Mediterranean are too low to be included in the analyses (Table 1).
Trento province has an elevation that ranges from 52 to 3,558 m asl, with about 77% of the province over 1,000 m asl. The province is forested up to treeline at about 2,000 m, above which it consists of Alpine meadows, rocky outcrops, scree fields, and open rock faces. Alpine chamois hunting is subdivided between 28 hunting districts, which are further subdivided into 209 municipal reserves (hunting management units).
Italy: Alpine Chamois Dataset
In Italy, chamois are culled with rifles from mid-September to mid-December. This time period encompasses a pre-rut period as well as the entire rut (Mason et al., 2011). Hunting is controlled through licenses issued by local wildlife boards. Area-wide hunting quotas are set for specific age classes in each sex. Data were collected on the undressed body mass (i.e., weighed without viscera and flowing blood), sex, age, and the harvesting date of 27,629 Alpine chamois (14,769 males and 12,860 females) harvested over 10 consecutive hunting seasons between 2007 and 2016. Age was estimated by counting horn growth annuli (Schröder and Von Elsner-Schak, 1985). Male age varied between 1 and 20 years, and female age between 1 and 21 years, respectively.
Slovenia: Roe Deer, Red Deer, and Chamois Datasets
In Slovenia, the roe deer rut starts around mid-July and finishes around mid-August. Male roe deer rifle hunting encompasses that period, extending from the beginning of May to the end of October. Data were collected on the undressed body mass, age category (yearlings, adults) and the harvesting date of all roe deer males culled throughout Slovenia over 11 consecutive hunting seasons between 2007 and 2017. Determination of age category of roe deer bucks was made by local hunting authorities using macroscopic inspection of dentition development of premolars and molars in the left hemimandibles accompanying each individual, which is a routine method enabling yearlings and adults to be distinguished reliably (Ratcliffe and Mayle, 1992; Pokorny and Jelenko Turinek, 2017). Because of uncertainty in age assessment of adult roe deer by macroscopic inspection of tooth wear (Hewison et al., 1999) all adults were pooled into one group.
Red deer stags were harvested with rifles from mid-August till the end of December. The rut typically starts in the mid-September and lasts for less than 1 month. Data were collected on the undressed body mass, age category (yearlings, 2–4-years, 5–9-years, and 10+ years old), and the harvesting date of all red deer stags shot over 11 consecutive hunting seasons between 2007 and 2017. Age category of red deer stags was estimated post-mortem on the basis of tooth wear of the left hemimandibles evaluated by experienced and authorized wildlife managers.
Chamois sample set included all adult males harvested in the same study period (2007–2017); also for this species, data on undressed body mass, age (assessed by counting horn grown annuli), and date of harvest were available in the Central Slovene hunting information system.
Data Analysis
Data collected from hunting-management districts were grouped with respect to main area (population) and year. We focused only on age classes of males that had reached social maturity and were fully involved in the rut. Threshold ages for social maturity, i.e., the age at which males are not only sexually mature but also able to sustain intersexual competition having reached full body development, were based on published data on rutting activities and hypophagia of male age classes (Mysterud et al., 2005). For roe deer, senescent individuals (assessed age >10 years) were discarded (Hewison et al., 2011). Male age classes, considered in our paper, were as follows: roe deer: 3–10 years old (Vanpé et al., 2008; Hewison et al., 2011); red deer >5 years old (Clutton-Brock, 1984; Mysterud et al., 2008); and chamois >5 years old (von Hardenberg et al., 2000; Corlatti et al., 2012). The final sample set considered for the analysis consisted of 71,420 ungulates (6,315 of which were chamois females, analyzed with the aim to remove seasonal effect of body-mass decline in this species), subdivided by areas provided in Table 1.
To determine reproductive investment, we quantified the reduction of body mass of males from the beginning to the end of the rut as a measure of the cost of reproduction. To compare body mass loss among different species and among populations of the same species, we standardized the protocol as follows: (i) a time series was created by computing the average (undressed) body mass of each consecutive day of the year; (ii) an intervention analysis (Box and Tiao, 1975) identified break points in the series, defining the beginning of the decline in body mass, which indicates the beginning of the rut; (iii) in each population, each animal’s weight was standardized as a percentage of the body mass observed at the beginning of the rut; (iv) populations with standardized body masses were grouped by synchronizing the beginning of the rut (aligning the time series to the onset of rut as a common start point). Note that the analysis in step (ii) comprises a group of techniques that aims to find changes in time series data, including changes in the overall trend or in the amplitude after an event occurred (intervention). In time series, data are often autocorrelated and there may be seasonality and high variability around the mean, confounding efforts to recognize changes in patterns. To take into account these properties, ARIMA models iteratively evaluate the likelihood of alternative models with different dates of changes of time series properties, finally finding the model that best explains the patterns.
All computations were conducted in the R system for statistical computing (R Development Core Team, 2019 – version 3.6.0). Intervention analysis was performed with package strucchange 1.5–1, that uses the Bayesian Information Criterion to select the best number of breakpoints in a time series (Zeileis et al., 2002).
Start and end dates of body-mass decline were slightly different among populations of the same species. Thus, population time series were aligned to the beginning of the rut (assumed, for our purposes, to be identified by the beginning of body mass decline) by using the mean value among populations, to preserve as much as possible of each time series (and of the aggregated series).
To facilitate comparisons across populations, body-mass values also were standardized. New values were represented by percentages of the average value of the body mass in the 15 days before the beginning of the rut. In this way, the initial standardized body mass for all populations was equal to 100, and body mass on a given day was expressed as a percentage of that initial value.
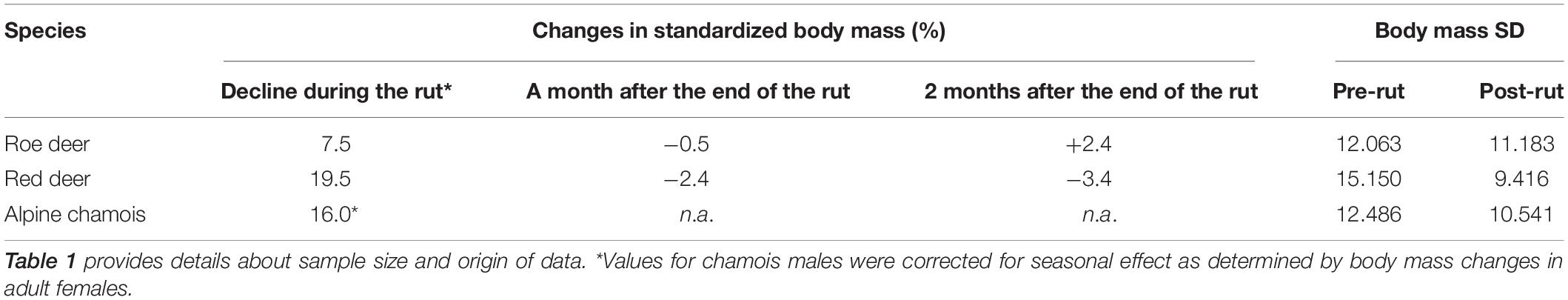
Standardized body masses and Julian dates enabled us to group all data for each species, creating a unified time series on which a further intervention analysis was used to identify the end point of body-mass declines. An index of the loss of body mass resulting from reproduction was estimated as the difference between 100 (initial standardized body mass) and average standardized body mass of individuals 15 days after the end of the weight decline. To describe the pattern of body mass after reproduction, for roe deer and red deer (chamois data were lacking) the same computation was made 1 and 2 months after the end of the rut.
Because the chamois rut takes place in the second half of autumn and a decline in body mass is observed in females resulting from seasonal rather than reproductive factors, we also estimated body-mass changes of adult females (for this species only). Body mass decline observed in chamois females was then subtracted from that of males, to consider only the reproductive component of body mass variation in males.
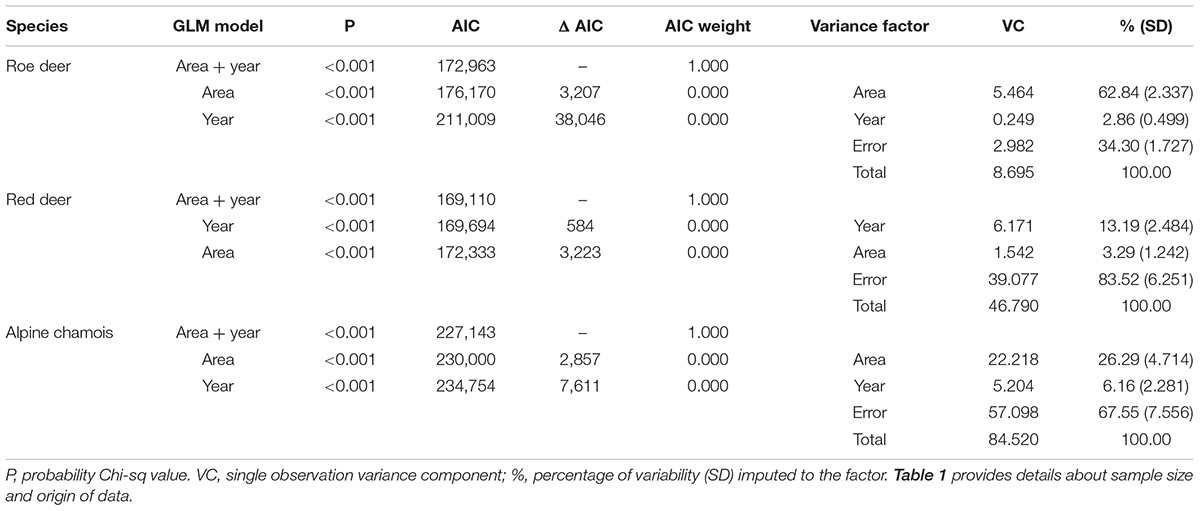
Environmental variability is important in shaping life-history traits in ungulates (Gaillard et al., 2003). Consequently, we estimated for each species the variance in body mass loss because of sample differences in areas and years. We estimated variability of body-mass loss in each area and year by resampling 1,000 times (R package “boot” 1.3–23 – Davison and Hinkley, 1997; Canty and Ripley, 2019) the initial and final sample of body mass measurements. To evaluate relative importance of area and year in shaping loss of body mass, we built afterward for each species three Generalized Linear Models. Two models included as fixed factors either area or year as single independent predictors, and the third one included both predictors. Model-selection used AIC and AIC weights (Burnham and Anderson, 2002). Finally, we preformed variance components analysis using the VCA package (version 1.4.0 – Searle et al., 1992; Schuetzenmeister and Dufey, 2019) that allows unbalanced designs in computing the variance of variance components.
Results
In the best time series model determined by intervention analysis, the trend in roe deer standardized body mass exhibited three break points (Figure 1). The first identified the beginning of the decline, on days 194–195 of the combined Julian date (13–14 July). At that time, observed mean body mass was 18.69 kg (SE = 0.029; n = 6,100). The second break-point corresponded to a steady change in the steepness of the decline on days 228–229 (16–17 August), when the observed mean body mass was 17.24 kg (SE = 0.030; n = 4,584). The third break-point, on days 265–266 (24–25 September), indicated the start of a period of fat gain, possibly related to storage and preparation for winter.
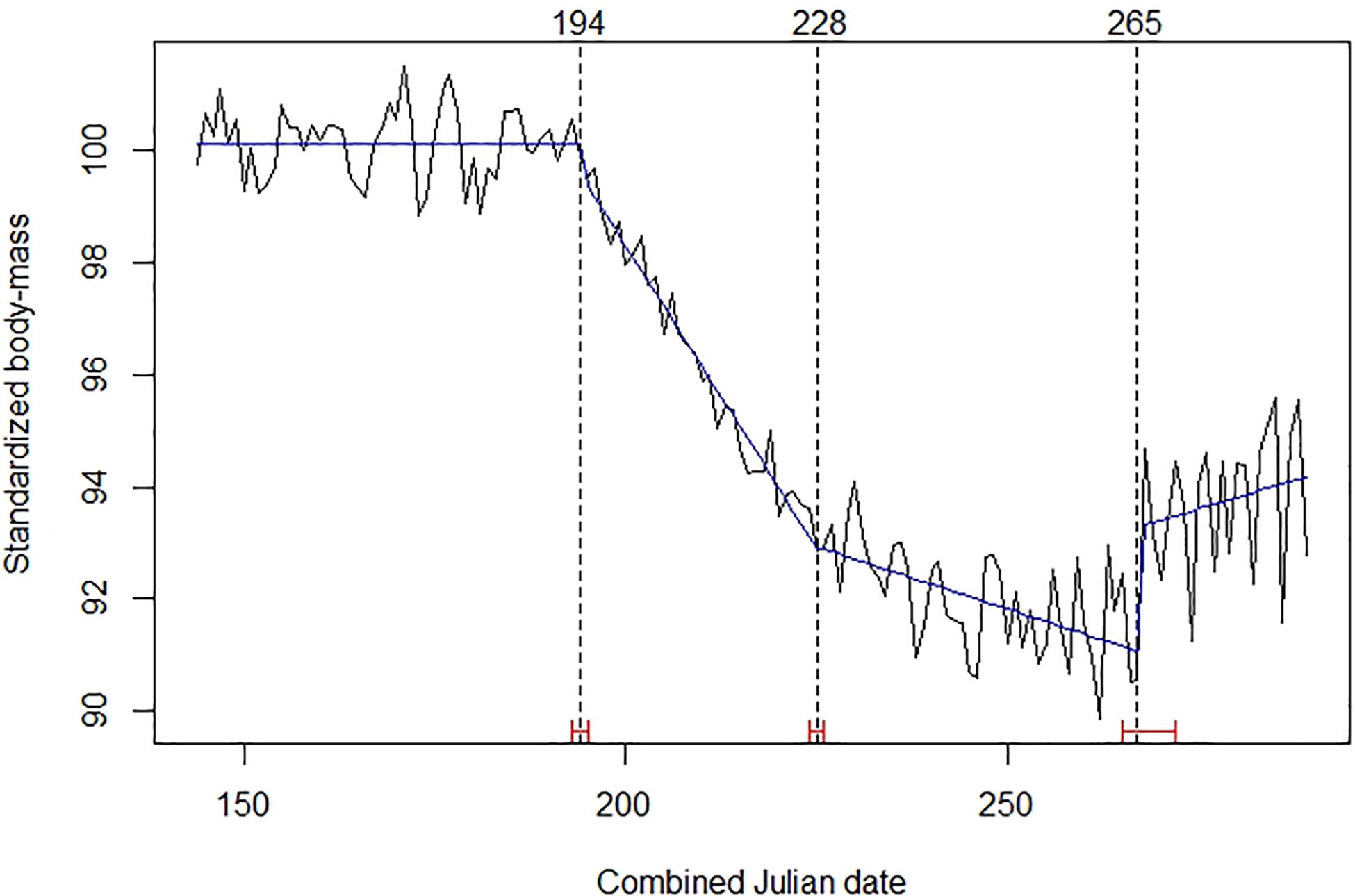
Figure 1. Mean daily standardized body mass of roe deer (black broken line), trends (blue line), Julian date of estimated structural changes (dashed lines, labeled at the top), and 95% confidence intervals of estimated structural change date (red bars). Table 1 provides details about sample size and origin of data.
A model with two break points provided the best explanation for the body mass time series of red deer (Figure 2). The first break point was on days 245–246 (2–3 September), when the trend became strongly negative. Red deer observed mean body mass at that first breakpoint was 163.0 kg (SE = 1.531; n = 293). The second break was on days 272–273 (29–30 September), when decline in body mass significantly reduced its steepness; mean body mass at that second breakpoint was 131.1 kg (SE = 0.756; n = 475).
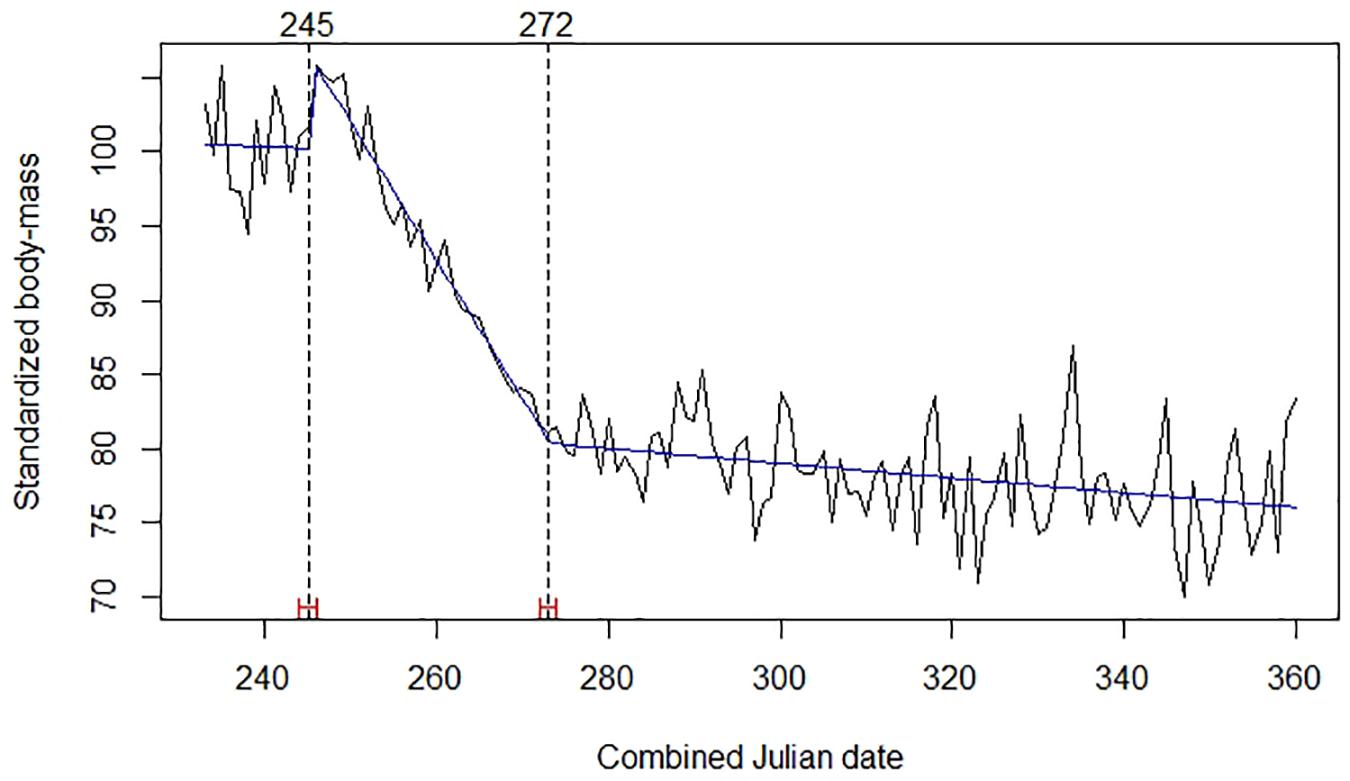
Figure 2. Mean daily standardized body mass of red deer (black broken line), trends (blue line), Julian date of estimated structural changes (dashed lines, labeled at the top), and 95% confidence intervals of estimated structural change date (red bars). Table 1 provides details about sample size and origin of data.
The best-fitting model of the time series for chamois body mass involved three break-points (Figure 3). Those of interest, because of their role in defining the end of the rut, were the last two points. The second was set on days 297–298 (24–25 October), at the start of a strong decline in body mass that was initially observed equal to 28.39 kg (SE = 0.131; n = 758) on average. The third break point was on days 343–344 (9–10 December), when the decline significantly reduced and chamois mean observed body mass was 21.36 kg (SE = 0.157; n = 366). Comparing time series of body mass for male and female chamois allowed us to estimate the seasonal reduction of body mass of 1.6 kg after the end of October also in females, corresponding to 7.5% of their standardized body mass (Figure 4). This weight loss was not considered as a reproductive cost for males; consequently, we excluded this percentage of body mass loss from calculations for males.
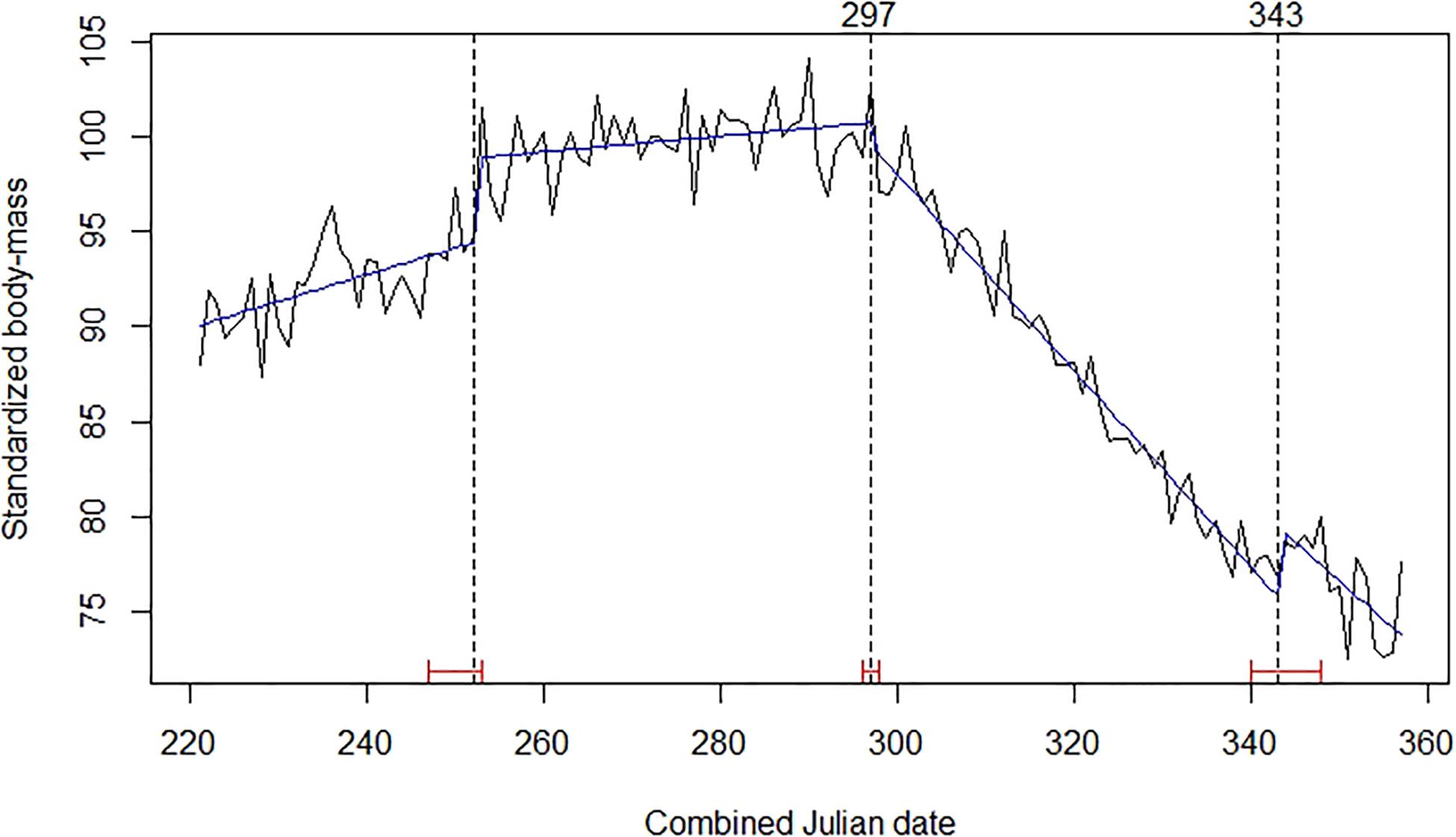
Figure 3. Mean daily standardized body mass of Alpine chamois (black broken line), trends (blue line), Julian date of estimated structural changes (dashed lines, labeled at the top), and 95% confidence intervals of estimated structural change date (red bars). Table 1 provides details about sample size and origin of data.
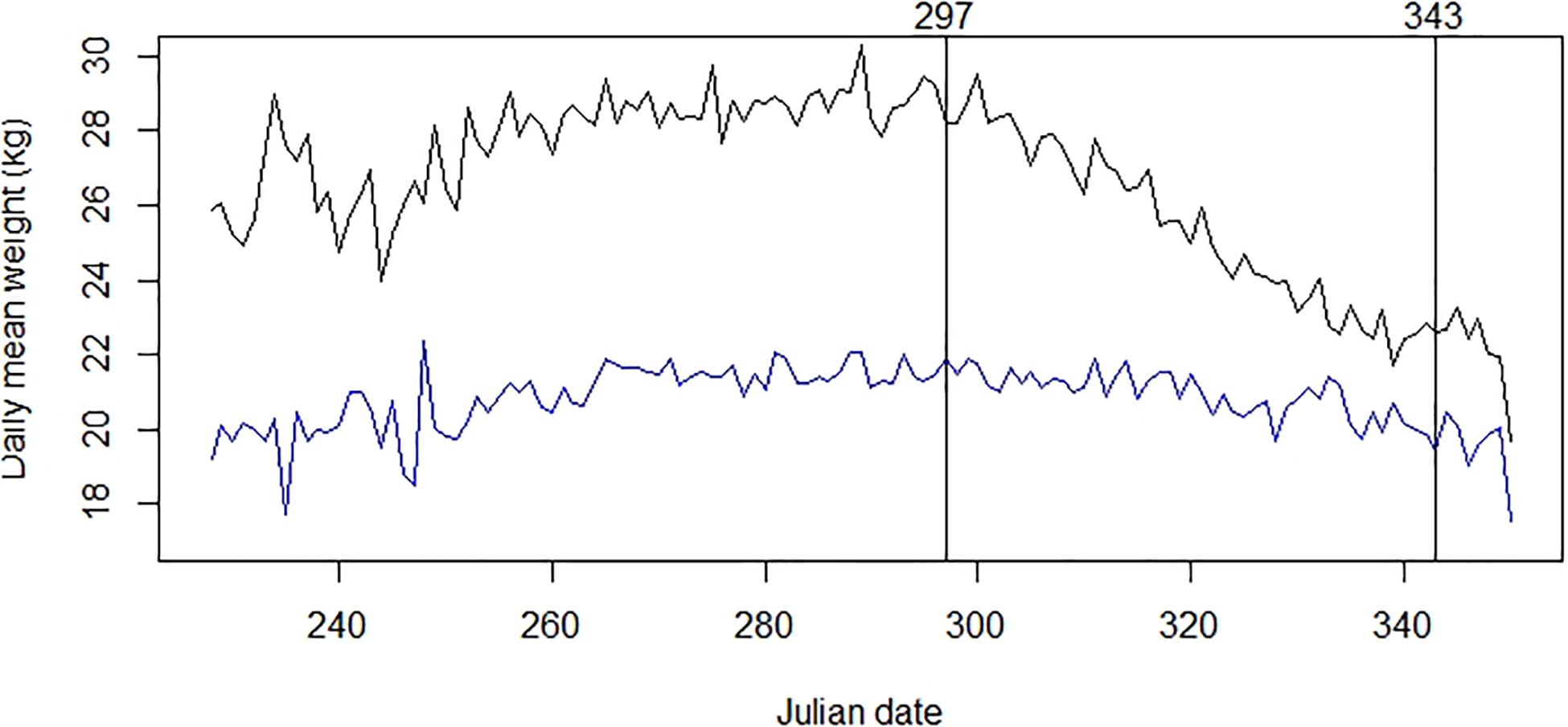
Figure 4. Males (black) and females (blue) chamois average daily body mass and rut period (defined by the two labeled black lines). Table 1 provides details about sample size and origin of data.
The decline in standardized body mass at the beginning and the end of the rut exhibited pronounced differences among the three species of ungulates. Decreases in body mass were the highest in red deer (19.5%), and the lowest in roe deer (7.5%). In roe deer, the body mass decrease was, on average, less than one-half that of chamois (16.0%) (Table 2). Body mass of red deer continued to decline 2 months after the rut (additional 3.4% of reduction). By contrast, 2 months after the rut, roe deer average body mass had increased by 2.4%.
Red deer showed the greatest differences in body-mass variability between the beginning and the end of the rut, while roe deer showed the smallest ones (Table 2). In red deer, body mass dispersion around the mean value was larger at the beginning of the rut than at the end. In chamois, the observed pattern was similar to red deer although differences were smaller. In roe deer, body mass variability was similar before and after the rut.
For the three species, area and year differed in their importance for explaining changes in proportional mass loss (Table 3). In roe deer, variability in body mass loss was mainly because of spatial differences, with differences between years explaining little of the variation in mass loss. Although slight effects of area and year were evident for both red deer and chamois, these were overwhelmed by residual variance for both species.
Discussion
The ecological and evolutionary drivers of the placement of a species on the capital-income breeding continuum have been the focus of considerable interest (Jönsson, 1997; Bonnet et al., 1998; Stephens et al., 2009). In this context, explanations have tended to focus on three broad classes of drivers: aspects of food supply, morphological or physiological constraints, and habitat characteristics (Stephens et al., 2009). Here, by contrast, we argue that a behavioral factor might favor the adoption of a particular capital or income breeding strategy. Specifically, we argue that high levels of male-male competition, resulting from high degrees of polygyny, are likely to favor a strong focus on competitive interactions during the mating period, with a consequent reduction in foraging. This pattern, in turn, indicates that high levels of polygyny will necessitate a heavy reliance on stored capital during the mating period. High levels of polygyny in our species also correspond to different group sizes during the mating season: red deer and Alpine chamois adult males can have access to groups of females, as females of these species, in particular, are social; by contrast, roe deer adult males can have access only to solitary females at any time, and inside their territory only. Our very large dataset of carcass weights of three common and widespread ungulate species, obtained from two European countries, is consistent with our hypothesis: male roe deer, a species exhibiting low levels of polygyny and therefore limited variance in lifetime reproductive success (Vanpé et al., 2008), lose a relatively small proportion of body mass during the rut, indicating a low reliance on stored energetic capital during that period; by contrast, male red deer and Alpine chamois, both of which are known to have either high levels of polygyny (red deer) or at least high male-male competition (both species) (Kramer, 1969; Clutton-Brock, 1984; Pemberton et al., 1992; Marshall et al., 1998; Garel et al., 2009; Rughetti and Festa-Bianchet, 2011; Corlatti and Bassano, 2014), show much larger levels of body-mass loss during the rut, suggesting a high reliance on stored energetic capital. We discuss these findings in relation to two main issues: the broader ecology and life-history of our focal species, and the limitations on identifying cause and effect in an observational life-history study, with potential directions for future research.
Capital and Income Breeding and the Life-Histories of Male Ungulates
Our hypothesis relating proximity to the capital end of the capital-income breeder continuum to the degree of polygyny of mating systems was entirely consistent with the outcomes of our comparison between the two deer species. Red deer showed a decrease in body weight during the rut that was 2.5-times larger than in roe deer. We argue that this is associated with a much larger variance in lifetime breeding success (Im) in red deer stags (Im ≥ 3.0) than in roe deer bucks (Im ∼ 0.75) (Pemberton et al., 1992; Marshall et al., 1998; Vanpé et al., 2008).
Alpine chamois bucks demonstrated a marked loss of body mass during the rut, losing about 16% of the initial weight, which is more than twice the mass loss experienced by roe deer bucks. In that sense, chamois seem to be closer to the capital rather than to the income end of the breeding continuum. This is surprising because, owing to their unbiased sex-specific survival (Rughetti and Festa-Bianchet, 2011; Corlatti et al., 2012), they are not considered to be strongly polygynic (Corlatti et al., 2013a). The high similarity of male and female body size, however, does not constitute a useful diagnostic of their level of polygyny. Indeed, in male chamois physical competition is related to agility and not just to body mass, which is the dominant factor for most deer species that use fighting strategies based on wresting or ramming (Kramer, 1969; Clutton-Brock et al., 1979; Festa-Bianchet et al., 1990). As a related example, horses are nearly monomorphic but are recognized to be highly polygynous (Berger, 1986; Rubenstein, 1986). Notably, despite the lack of dimorphism in the average body masses of male and female chamois, evidence exists of seasonal changes in the degree of dimorphism. Specifically, male chamois body weight is about 40% greater than that of females before the rut but that difference declines to only about 6% in January (Garel et al., 2009; Rughetti and Festa-Bianchet, 2011). This is probably because chamois bucks have to gain energy in order to cope with intense energy expenditure of a late autumn rut in a harsh mountain environment.
However, seasonal change in the degree of body-mass dimorphism in Alpine chamois is also consistent with the potential for high energy expenditure by males, linked to a higher level of polygyny than expected from overall levels of dimorphism alone. The rationale behind the link between the level of polygyny and condition of capital-income breeders is provided by the differential opportunities of mating for adult males in scarcely vs. highly polygynic mating systems. More mating opportunities imply higher competition and the need for higher energy expenditure to cope with intrasexual conflicts that, in turn, lead to a capital breeder strategy relying on long term energy storage. By contrast, limited mating opportunities do not justify year-long (or season-long) accumulation of large amounts of stored energy that, for various reasons, can be detrimental to survival (see, for instance, Varpe and Ejsmond, 2018). Measures of lifetime breeding success for chamois would help to bolster confidence in this suggestion.
In mammals, the energy costs for males of acquiring mating opportunities can often exceed those of lactating females (Lane et al., 2010). Energy storage to meet the costs of future reproduction could also represent an advanced payment of reproductive costs (e.g., in term of predation risk) that can be justified only if the outcome overcomes the investment. A further difference between males belonging to opposite ends of the capital-income breeder continuum seems to be the seasonal dynamic of the pre- and post-rut variance in body mass variability among males. For income breeders (i.e., roe deer males), there was a minimal change in the standard deviation of body mass before and after the rut, demonstrating that rutting activities did not modify body-mass variance among adult males. This outcome is consistent with the delayed implantation that characterizes this species, allowing the choice of optimal timing for mating, placed in the least-limiting season (Sandell, 1990). In contrast, the capital breeding red deer stags exhibited a considerable decrease of variance in body mass at the end of the rut, indicating that differential investment by males of different body mass and quality led to a more even condition among them at the end of the rut. This result implies that there is some body mass threshold, below which stags cannot decrease their weight without compromising their survival during the following winter. Although the effect is less pronounced than in red deer, the same seems to be true for Alpine chamois.
Where possible (i.e., within both deer species), we also evaluated changes in body mass after the intense effort connected with the rut. We documented that the capital-breeding red deer continued to lose body mass after the rut, whilst the income-breeding roe deer males were able to recover part of the loss suffered during the rut within 2 months of the end of the mating season. This result is likely to be related to two different factors: (i) the timing of the rut that, in the case of roe deer, occurred in the middle of summer, in contrast to red deer that, after the rut, have to face a limiting time both in resources and climate like the last part of autumn and then winter; and (ii) the lower level of energy expenditure of roe deer, leading to a greater ability to recover rut losses.
The reduced post-rut variability among males in capital breeders and a fast recovery of body mass of an income breeder can be interpreted in the light of the differential ability of capital and income breeders to cope with a variable environment in a number of taxa (Pélisson et al., 2012). Income breeders seem to be more susceptible to sudden environmental changes, and rutting in summer seems to be a strategy that allows roe deer males efficiently to buffer the loss of body condition during reproduction.
Our models to explain variation in male body mass loss among areas and years suggest that interindividual differences are the major factors for red deer and chamois, whilst area is the dominant factor dictating weight loss for roe deer (Gaillard et al., 1993; Linnell and Andersen, 1998; Raganella-Pelliccioni et al., 2007; Plard et al., 2014). This is consistent with the idea that capital breeders are less dependent on environmental factors linked to different resource availability in space (area) or time (year) than income breeders like roe deer. As noted by Kerby and Post (2013), the capital-breeder strategy seems to be more suitable to cope with a changing environment as it relies on long-term accumulation of energy rather than on the immediate conditions preceding the start and the development of rutting activities. Long-term accumulation gives the potential to buffer against sudden losses of resources due to extreme climatic events, and therefore constitutes a better strategy to guarantee the sustainability of high energy expenditure linked to strong polygyny and the connected high opportunity of gaining reproduction success. Moreover, capital breeders seem more able to cope with human induced disturbance, a further element of environmental unpredictability (for evidence that the effect of disturbance is greater for income than capital breeders, see also McHuron et al., 2017).
Limitations and Future Directions
Our findings are consistent with a link between capital and income breeding in males, and the degree of polygyny they express. Our results challenge ecologists to consider mating systems as a further axis of variation that could push species toward one or the other end of the capital-income spectrum. Inevitably, however, our findings do not “prove” a causal link between mating systems and degree of reliance on stored capital for male mating. This is because of both the nature of correlational data vs. experimentation, and the complexity of life-history systems.
Although our sample sizes for carcass weights are large, they represent transverse observational data on only three species. Despite the difficulty of obtaining large sample sizes, longitudinal data are often preferred for life-history studies (Gaillard et al., 2003). In this instance, data on individuals that breed in some years but not in others would be ideal for estimating the true body mass loss associated with reproduction alone. Nevertheless, such data would be difficult to obtain in the wild, as most male ungulates attempt to breed in all years (Loison et al., 1999), thereby incurring some costs. In captivity, it would be possible to conduct experimental manipulations of the potential to breed, but this would be confounded by the very different food regimes available in those situations. All metrics would be improved by simultaneous observations of the extent of feeding, which would enable estimates of the relative contributions of stores and intake to finance the costs of reproduction. In general, however, insights into the costs of reproduction have been as a rule obtained from transverse data in the past (Mysterud et al., 2008; Mason et al., 2011); because of a very large sample set used in our study, such insights are likely sufficiently accurate to characterize the broad differences between populations and species of interest.
Here, we presented data on three heavily hunted species for which large data sets are available. These include only one species toward the income breeding end of the spectrum and two toward the capital breeding extreme. Certainly, these species vary in many aspects of ecology and life-history, not just in their degree of polygyny. This rightly prevents us from attributing cause and effect in the system. Nevertheless, our main purpose was to demonstrate that the hypothesis on the relation between the level of polygyny and position of ungulate males within the capital-income breeding spectrum is consistent with observation in three data-rich species. Further research could take this forward on several fronts. First, other species are also heavily hunted and could yield similar data (e.g., moose, fallow deer, mouflon Ovis gmelini musimon, reindeer, and wild boar Sus scrofa among European ungulates). It would be interesting to determine whether those species, broadly characterized as showing high or low degrees of polygyny, are also consistent with our expectations. With a large enough sample of populations and species, and given a measure of variance in lifetime reproductive success of males within them, more quantitative phylogenetic comparisons would also be possible. Given the likely link between seasonal gluts of food availability and capital breeding as well as our posited link between capital breeding and polygyny, we might expect populations in more seasonal environments to exhibit greater polygyny and greater reliance on stored capital as substantial seasonal differences exist in energy financing opportunities. Finally, although life-history traits do not evolve in strictly linear causal chains (Stephens et al., 2009), theoretical models can shed light on the correlations expected between capital and income breeding and other aspects of organism-environment interactions (Houston et al., 2007; Stephens et al., 2014). Our purposes would be well-served by models that alter only female dispersion, thus modifying an important part of the potential for polygyny (Emlen and Oring, 1977), and allow variation in energy storage strategies as well as the extent of polygyny. Distinct emergent optima arising from covariation in these two attributes would be strongly supportive of the link that we propose in this paper.
Conclusion
Here, we suggest that – owing to the high demands of time and energy that polygynous males face during the reproductive period – it is likely that males in highly polygynous species are more likely to be reliant on capital breeding than are those in relatively monogamous species. Our data on seasonal patterns of body mass change in three ungulate species are consistent with this hypothesis, indicating that mating systems should be considered as a further factor driving variation on the capital-income breeding spectrum. Both empirical and theoretical approaches should cast further light on the validity of our hypothesis, which should provide new insights and help advance the field of ungulate ecology.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
Ethical review and approval was not required for the animal study because all individuals used in the study were hunted during the regular hunting activity prescribed by the state of Slovenia and Italy within the yearly hunting management plans. We used only data on dead individuals therefore no animal was shot or killed by any other means for the purposes of the research.
Author Contributions
MA, BP, EM, and RC conceived and planned the experiment. EM analyzed the data. RC, KF, and AA collected and prepared data. MA, EM, RC, BP, and PS contributed to the interpretation of the results. MA and EM took the lead in writing the manuscript. All authors provided critical feedback and helped shape the research, analysis, and manuscript.
Funding
Slovene co-authors acknowledge the financial support from the Ministry of Agriculture, Forestry and Food (project V4–1825) and the Slovenian Research Agency (ARRS), which funded KF and BP through the programme group P4–0107. ARRS was also foreseen to fund the Open Access publication fee.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to the Hunters Association of Slovenia for developing on-line available hunting information system enabling us the access to all needed data on all harvested ungulates in Slovenia, and to the Trento Province and the Trentino Hunters’ Association for supplying Italian chamois data. We also thank the associate editor, RB, and the reviewers, LZ, and PK, for very helpful comments improving the first version of the manuscript.
Footnotes
References
Alexander, R. D., Hoogland, J. L., Howard, R. D., Noonan, K. M., and Sherman, P. W. (1979). “Sexual dimorphisms and breeding systems in pinnipeds, ungulates, primates and humans,” in Evolutionary Biology and Human Social Behavior: An Anthropological Perspective, eds N. A. Chagnon and W. Irons (Scituate, MA: Duxbury Press), 402–435.
Andersen, R., Gaillard, J. M., Linnell, J. D., and Duncan, P. (2000). Factors affecting maternal care in an income breeder, the European roe deer. J. Anim. Ecol. 69, 672–682. doi: 10.1046/j.1365-2656.2000.00425.x
Apollonio, M., and Di Vittorio, I. (2004). Feeding and reproductive behaviour in fallow bucks (Dama dama). Naturwissenschaften 91, 579–584. doi: 10.1007/s00114-004-0574-0
Barboza, P. S., Hartbauer, D. W., Hauer, W. E., and Blake, J. E. (2004). Polygynous mating impairs body condition and homeostasis in male reindeer (Rangifer tarandus tarandus). J. Comp. Physiol. B 174, 307–317. doi: 10.1007/s00360-004-0416-6
Berger, J. (1986). Wild Horses of the Great Basin: Social Competition and Population Size. Chicago: The University of Chicago Press.
Bergman, C. M., Fryxell, J. M., Gates, C. C., and Fortin, D. (2001). Ungulate foraging strategies: energy maximizing or time minimizing? J. Anim. Ecol. 70, 289–300. doi: 10.1111/j.1365-2656.2001.00496.x
Bobek, B., Perzanowski, K., and Weiner, J. (1990). Energy expenditure for reproduction in male red deer. J. Mammal. 71, 230–232. doi: 10.2307/1382171
Bonnet, X., Bradshaw, D., and Shine, R. (1998). Capital versus income breeding: an ectothermic perspective. Oikos 83, 333–342. doi: 10.2307/3546846
Box, G. E., and Tiao, G. C. (1975). Intervention analysis with applications to economic and environmental problems. ıJ. Am. Stat. Assoc. 70, 70–79. doi: 10.1080/01621459.1975.10480264
Boyd, I. L. (2000). State-dependent fertility in pinnipeds: contrasting capital and income breeders. Funct. Ecol. 14, 623–630. doi: 10.1046/j.1365-2435.2000.t01-1-00463.x
Brivio, F., Grignolio, S., and Apollonio, M. (2010). To feed or not to feed? Testing different hypotheses on rut−induced hypophagia in a Mountain ungulate. Ethology 116, 406–415. doi: 10.1111/j.1439-0310.2010.01753.x
Burnham, K. P., and Anderson, D. R. (2002). Model Section and Multimodel Inferences: A Practical-Theoretic Approach, 2nd Edn. New York, NY: Springer-Verlag.
Bužan, E., Bryja, J., Zemanová, B., and Kryštufek, B. (2013). Population genetics of chamois in the contact zone between the Alps and the Dinaric mountains: uncovering the role of habitat fragmentation and past management. Cons. Genet. 14, 401–412. doi: 10.1007/s10592-013-0469-8
Canty, A., and Ripley, B. D. (2019). Boot: Bootstrap r (s-plus) Functions. R Package Version 1.3-23. Cambridge: Cambridge University Press.
Carranza, J., Alvarez, F., and Redondo, T. (1990). Territoriality as a mating strategy in red deer. Anim. Behav. 40, 79–88. doi: 10.1016/s0003-3472(05)80667-0
Clutton-Brock, T. H. (1984). Reproductive effort and terminal investment in iteroparous animals. Am. Nat. 123, 212–229. doi: 10.1086/284198
Clutton-Brock, T. H. (1989). Mammalian mating systems. Proc. R. Soc. Lond. Ser. B 236, 339–372. doi: 10.1098/rspb.1989.0027
Clutton-Brock, T. H., Albon, S. D., Gibson, R. M., and Guinness, F. E. (1979). The logical stag: adaptive aspects of fighting in red deer (Cervus elaphus L.). Anim. Behav. 27, 211–225. doi: 10.1016/0003-3472(79)90141-6
Clutton-Brock, T. H., Guinness, F., and Albon, S. (1982). Red Deer Behaviour and Ecology of Two Sexes. Cambridge: Cambridge University Press.
Coltman, D. W., Festa-Bianchet, M., Jorgenson, J. T., and Strobeck, C. (2002). Age-dependent sexual selection in bighorn rams. Proc. R. Soc. Lond. B 269, 165–172. doi: 10.1098/rspb.2001.1851
Coltman, D. W., Pilkington, J. G., Smith, J. A., and Pemberton, J. M. (1999). Parasite−mediated selection against inbred Soay sheep in a free−living island population. Evolution 53, 1259–1267. doi: 10.1111/j.1558-5646.1999.tb04538.x
Connan, M., Dilley, B. J., Whitehead, T. O., Davies, D., McQuaid, C. D., and Ryan, P. G. (2019). Multidimensional stable isotope analysis illuminates resource partitioning in a sub−Antarctic island bird community. Ecography 42, 1948–1959. doi: 10.1111/ecog.04560
Corlatti, L., and Bassano, B. (2014). Contrasting alternative hypotheses to explain rut-induced hypophagia in territorial male chamois. Ethology 120, 32–41. doi: 10.1111/eth.12177
Corlatti, L., Bassano, B., Valencak, T. G., and Lovari, S. (2013a). Foraging strategies associated with alternative reproductive tactics in a large mammal: foraging strategies of male chamois. J. Zool. 291, 111–118. doi: 10.1111/jzo.12049
Corlatti, L., Béthaz, S., von Hardenberg, A., Bassano, B., Palme, R., and Lovari, S. (2012). Hormones, parasites and male mating tactics in Alpine chamois: identifying the mechanisms of life history trade-offs. Anim. Behav. 84, 1061–1070. doi: 10.1016/j.anbehav.2012.08.005
Corlatti, L., Caroli, M., Pietrocini, V., and Lovari, S. (2013b). Rutting behaviour of territorial and nonterritorial male chamois: is there a home advantage? Behav. Proc. 92, 118–124. doi: 10.1016/j.beproc.2012.11.008
Davison, A. C., and Hinkley, D. V. (1997). Bootstrap Methods and Their Applications. Cambridge: Cambridge University Press.
Debeffe, L., Focardi, S., Bonenfant, C., Hewison, A. J. M., Morellet, N., Vanpé, C., et al. (2014). A one night stand? Reproductive excursions of female roe deer as a breeding dispersal tactic. Oecologia 176, 431–443. doi: 10.1007/s00442-014-3021-8
Drent, R. H., and Daan, S. (1980). The prudent parent: energetic adjustments in avian breeding. Ardea 68, 225–252. doi: 10.5253/arde.v68.p225
Emlen, S. T., and Oring, L. W. O. (1977). Ecology, sexual selection, and the evolution of mating systems. Science 197, 215–223. doi: 10.1126/science.327542
Festa-Bianchet, M., Apollonio, M., Mari, F., and Rasola, G. (1990). Aggression among lekking male fallow deer (Dama dama): territory effects and relationship with copulatory success. Ethology 85, 236–246. doi: 10.1111/j.1439-0310.1990.tb00403.x
Flajšman, K. (2017). Effects of Individual, Population and Environmental Factors on Parameters of Reproductive Success of Female Roe Deer (Capreolus capreolus L.). Doctoral dissertation, University of Ljubljana, Biotechnical faculty, Ljubljana.
Gaillard, J. M., Delorme, D., Boutin, J. M., Van Laere, G., Boisaubert, B., and Pradel, R. (1993). Roe deer survival patterns: a comparative analysis of contrasting populations. J. Anim. Ecol. 62, 778–791. doi: 10.2307/5396
Gaillard, J. M., Loison, A., and Toïgo, C. (2003). “Variation in life history traits and realistic population models for wildlife management,” in Animal Behavior and Wildlife Conservation, eds M. Festa-Bianchet and M. Apollonio (Washington, DC: Island Press), 115–132.
Garel, M., Loison, A., Jullien, J. M., Dubray, D., Maillard, D., and Gaillard, J. M. (2009). Sex-specific growth in Alpine chamois. J. Mammal. 90, 954–960. doi: 10.1644/08-MAMM-A-287.1
Hewison, A. J. M., Gaillard, J. M., Delorme, D., Van Laere, G., Amblard, T., and Klein, F. (2011). Reproductive constraints, not environmental conditions, shape the ontogeny of sex-specific mass-size allometry in roe deer. Oikos 120, 1217–1226. doi: 10.1111/j.1600-0706.2011.19316x
Hewison, A. J. M., Vincent, J. P., Angibault, J. M., Delorme, D., Van Laere, G., and Gaillard, J. M. (1999). Tests of estimation of age from tooth wear on roe deer of known age: variation within and among populations. Can. J. Zool. 77, 58–67. doi: 10.1139/z98-183
Houston, A. I., Stephens, P. A., Boyd, I. L., Harding, K. C., and McNamara, J. M. (2007). Capital or income breeding? A theoretical model of female reproductive strategies. Behav. Ecol. 18, 241–250. doi: 10.1093/beheco/arl080
Jaatinen, K., Öst, M., and Hobson, K. A. (2016). State-dependent capital and income breeding: a novel approach to evaluating individual strategies with stable isotopes. Front. Zool. 13:24. doi: 10.1186/s12983-016-0157-x
Jönsson, K. (1997). Capital and income breeding as alternative tactics of resource use in reproduction. Oikos 78, 57–66. doi: 10.2307/3545800
Kerby, J., and Post, E. (2013). Capital and income breeding traits differentiate trophic match–mismatch dynamics in large herbivores. Phil. Trans. R. Soc. B 368:20120484. doi: 10.1098/rstb.2012.0484
Kojola, I. (1991). Influence of age on the reproductive effort of male reindeer. J. Mammal. 72, 208–210. doi: 10.2307/1382001
Komers, P. E., Messier, F., Flood, P. F., and Gates, C. C. (1994). Reproductive behavior of male wood bison in relation to progesterone level in females. J. Mammal. 75, 757–765. doi: 10.2307/1382527
Kramer, A. (1969). Soziale organisation und Sozialverhalten einer gemspopulation Rupicapra rupicapra der Alpen. Z. Tierpsychol. 26, 889–964. doi: 10.1111/j.1439-0310.1969.tb01983.x
Kurt, F. (1991). Das Reh in der Kulturlandschaft. Sozialverhalten und Ökologie eines Anpassers. Hamburg: Verlag Paul Parey.
Lane, J. E., Boutin, S., Speakman, J. R., and Humphries, M. M. (2010). Energetic costs of male reproduction in a scramble competition mating system. J. Anim. Ecol. 79, 27–34. doi: 10.1111/j.1365-2656.2009.01592.x
Lieberg, O., Johansson, A., Andersen, R., and Linnell, J. (1998). “Mating system, mating tactics and the function of male territoriality in roe deer,” in The European Roe Deer: The Biology of Success, eds R. Andersen, P. Duncan, and J. Linnell (Oslo: Scandinavian University Press), 221–256.
Linnell, J. D. C., and Andersen, R. (1998). Timing and synchrony of birth in a hider species, the roe deer Capreolus capreolus. J. Zool. 244, 497–504. doi: 10.1111/j.1469-7998.1998.tb00055.x
Loison, A., Gaillard, J. M., Pélabon, C., and Yoccoz, N. G. (1999). What factors shape sexual size dimorphism in ungulates? Evol. Ecol. Res. 1, 611–633.
Lovenčak, F. (1999). Naravnogeografske Značilnosti kot Možnost Razvoja Slovenije. Ljubljana: Univerza v Ljubljani.
Marshall, T. C., Slate, J., Kruuk, L. E. B., and Pemberton, J. M. (1998). Statistical confidence for likelihood−based paternity inference in natural populations. Mol. Ecol. 7, 639–655. doi: 10.1046/j.1365-294x.1998.00374.x
Mason, T. H. E., Chirichella, R., Richards, S. A., Stephens, P. A., Willis, S. G., and Apollonio, M. (2011). Contrasting life histories in neighbouring populations of a large mammal. PLoS One 6:e28002. doi: 10.1371/journal.pone.0028002
McElligott, A. G., Naulty, F., Clarke, W. V., and Hayden, T. J. (2003). The somatic cost of reproduction: what determines reproductive effort in prime-aged fallow bucks? Evol. Ecol. Res. 5, 1239–1250. doi: 10.5167/uzh-402
McHuron, E. A., Costa, D. P., Schwarz, L., and Mangel, M. (2017). State-dependent behavioural theory for assessing the fitness consequences of anthropogenic disturbance on capital and income breeders. Methods Ecol. Evol. 8, 552–560. doi: 10.1111/2041-210X.12701
Milner, J. M., Alexander, J., and Griffin, C. (2002). A Highland Deer Herd and Its Habitat. London: Red Lion House.
Miquelle, D. G. (1990). Why don’t bull moose eat during the rut? Behav. Ecol. Sociobiol. 27, 145–151. doi: 10.1007/BF00168458
Mysterud, A., Bonenfant, C., Loe, L. E., Langvatn, R., Yoccoz, N. G., and Stenseth, N. C. (2008). Age-specific feeding cessation in male red deer during rut. J. Zool. 275, 407–412. doi: 10.1111/j.1469-7998.2008.00453.x
Mysterud, A., Holand, Ø, Røed, K. H., Gjøstein, H., Kumpula, J., and Nieminen, M. (2003). Effects of age, density and sex ratio on reproductive effort in male reindeer (Rangifer tarandus). J. Zool. 261, 341–344. doi: 10.1017/S0952836903004114
Mysterud, A., Langvatn, R., and Stenseth, N. C. (2004). Patterns of reproductive effort in male ungulates. J. Zool. 264, 209–215. doi: 10.1017/S0952836904005618
Mysterud, A., Solberg, E. J., and Yoccoz, N. G. (2005). Ageing and reproductive effort in male moose under variable levels of intrasexual competition. J. Anim. Ecol. 74, 742–754. doi: 10.1111/j.1365-2656.2005.00965.x
Pélisson, P. F., Bel-Venner, M. C., Rey, B., Burgevin, L., Martineau, F., Fourel, F., et al. (2012). Contrasted breeding strategies in four sympatric sibling insect species: when a proovigenic and capital breeder copes with a stochastic environment. Funct. Ecol. 26, 198–206. doi: 10.1111/j.1365-2435.2011.01925.x
Pelletier, F., Page, K. A., Ostiguy, T., and Festa−Bianchet, M. (2005). Fecal counts of lungworm larvae and reproductive effort in bighorn sheep, Ovis canadensis. Oikos 110, 473–480. doi: 10.1111/j.0030-1299.2005.14120.x
Pemberton, J. M., Albon, S. D., Guinness, F. E., Clutton-Brock, T. H., and Dover, G. A. (1992). Behavioral estimates of male mating success tested by DNA fingerprinting in a polygynous mammal. Behav. Ecol. 3, 66–75. doi: 10.1093/beheco/3.1.66
Plard, F., Gaillard, J. M., Coulson, T., Hewison, A. M., Delorme, D., Warnant, C., et al. (2014). Long−lived and heavier females give birth earlier in roe deer. Ecography 37, 241–249. doi: 10.1111/j.1600-0587.2013.00414.x
Pokorny, B., and Jelenko Turinek, I. (2017). Čeljustnice Prostoživečih Parkljarjev. Ljubljana: Lovska zveza Slovenije.
R Development Core Team (2019). R: A Language and Environment for Statistical Computing. Version 3.6.0. Vienna: R Foundation for Statistical Computing.
Raganella-Pelliccioni, E., Scremin, M., and Toso, S. (2007). Phenology and synchrony of roe deer breeding in northern Italy. Acta Theriol. 52, 95–100. doi: 10.1007/BF03194204
Ratcliffe, P. R., and Mayle, B. A. (1992). Age determination of roe deer. For. Comm. Bull. 105, 26–28.
Rubenstein, D. I. (1986). “Ecology and sociality in horses and zebras,” in Ecological Aspects of Social Evolution: Birds and Mammals, eds D. I. Rubenstein and R. W. Wrangham (Princeton: Princeton University Press), 282–302. doi: 10.2307/j.ctt7zvwgq.17
Rughetti, M., and Festa-Bianchet, M. (2011). Seasonal changes in sexual size dimorphism in northern chamois: sexual dimorphism. J. Zool. 284, 257–264. doi: 10.1111/j.1469-7998.2011.00800.x
Sandell, M. (1990). The evolution of seasonal delayed implantation. Q. Rev. Biol. 65, 23–42. doi: 10.1086/416583
Schröder, W., and Von Elsner-Schak, I. (1985). “Correct age determination in chamois,” in The Biology and Management of Mountain Ungulates, ed. S. Lovari (London: Croom Helm), 65–70.
Schuetzenmeister, A., and Dufey, F. (2019). VCA: Variance Components Analysis. R package version 1.4.0. Available online at: https://cran.r-project.org/web/packages/VCA/VCA.pdf
Soulsbury, C. D. (2019). Income and capital breeding in males: energetic and physiological limitations on male mating strategies. J. Exp. Biol. 222:jeb184895. doi: 10.1242/jeb.184895
Stephens, P. A., Boyd, I. L., McNamara, J. M., and Houston, A. I. (2009). Capital breeding and income breeding: their meaning, measurement, and worth. Ecology 90, 2057–2067. doi: 10.1890/08-1369.1
Stephens, P. A., Houston, A. I., Harding, K. C., Boyd, I. L., and McNamara, J. M. (2014). Capital and income breeding: the role of food supply. Ecology 95, 882–896. doi: 10.1890/13-1434.1
Vanpé, C., Gaillard, J. M., Morellet, N., Kjellander, P., Liberg, O., Delorme, D., et al. (2009). Age-specific variation in male breeding success of a territorial ungulate species, the European roe deer. J. Mammal. 90, 661–665. doi: 10.1644/08-MAMM-A-137R.1
Vanpé, C., Kjellander, P., Galan, M., Cosson, J. F., Aulagnier, S., Liberg, O., et al. (2008). Mating system, sexual dimorphism, and the opportunity for sexual selection in a territorial ungulate. Behav. Ecol. 19, 309–316. doi: 10.1093/beheco/arm132
Varpe, Ø, and Ejsmond, M. J. (2018). Trade-offs between storage and survival affect diapause timing in capital breeders. Evol. Ecol. 32, 623–641. doi: 10.1007/s10682-018-9961-4
von Hardenberg, A., Bassano, B., Peracino, A., and Lovari, S. (2000). Male Alpine chamois occupy territories at hotspots before the mating season. Ethology 106, 617–630. doi: 10.1046/j.1439-0310.2000.00579.x
Williams, C. T., Klaassen, M., Barnes, B. M., Buck, C. L., Arnold, W., Giroud, S., et al. (2017). Seasonal reproductive tactics: annual timing and the capital-to-income breeder continuum. Phil. Trans. R. Soc. B 372:20160250. doi: 10.1098/rstb.2016.0250
Willisch, C. S., and Ingold, P. (2007). Feeding or resting? The strategy of rutting male Alpine chamois. Ethology 113, 97–104. doi: 10.1111/j.1439-0310.2006.01301.x
Yoccoz, N. G., Mysterud, A., Langvatn, R., and Stenseth, N. C. (2002). Age- and density-dependent reproductive effort in male red deer. Proc. R. Soc. Lond. Ser. B 269, 1523–1529. doi: 10.1098/rspb.2002.2047
Keywords: capital-income breeding, male reproductive investment, Capreolus capreolus, Cervus elaphus, Rupicapra rupicapra
Citation: Apollonio M, Merli E, Chirichella R, Pokorny B, Alagić A, Flajšman K and Stephens PA (2020) Capital-Income Breeding in Male Ungulates: Causes and Consequences of Strategy Differences Among Species. Front. Ecol. Evol. 8:521767. doi: 10.3389/fevo.2020.521767
Received: 19 December 2019; Accepted: 26 August 2020;
Published: 16 September 2020.
Edited by:
R. Terry Bowyer, University of Alaska Fairbanks, United StatesReviewed by:
Lin Zhang, Hangzhou Normal University, ChinaPetter Kjellander, Swedish University of Agricultural Sciences, Sweden
Copyright © 2020 Apollonio, Merli, Chirichella, Pokorny, Alagić, Flajšman and Stephens. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Boštjan Pokorny, bostjan.pokorny@vsvo.si; bostjan.pokorny@gmail.com
†These authors have contributed equally to this work
 Marco Apollonio
Marco Apollonio Enrico Merli
Enrico Merli Roberta Chirichella
Roberta Chirichella Boštjan Pokorny
Boštjan Pokorny Ajša Alagić4
Ajša Alagić4  Katarina Flajšman
Katarina Flajšman