Dietary Acid Load but Not Mediterranean Diet Adherence Score Is Associated With Metabolic and Cardiovascular Health State: A Population Observational Study From Northern Italy
- 1Department of Chemical and Pharmaceutical and Agricultural Sciences, University of Ferrara, Ferrara, Italy
- 2Department of Translational Medicine, University of Ferrara, Ferrara, Italy
- 3University Hospital of Ferrara Arcispedale Sant'Anna, Ferrara, Italy
- 4Department of Medical Surgical and Health Sciences, Clinica Medica ASUGI, University of Trieste, Trieste, Italy
- 5Hospital Pharmacy, Cattinara Hospital, Azienda Sanitaria Universitaria Giuliano Isontina, Trieste, Italy
- 6Department of Medicine, University of Udine, Udine, Italy
- 7Institute for Kinesiology Research, Science and Research Center of Koper, Koper, Slovenia
Diet plays a pivotal role in shaping the trajectory of chronic diseases. In this regard, the Mediterranean diet has been widely shown to exert beneficial effects on cardiometabolic health. On the contrary, the Western diet, which has also been reported to be an acidogenic dietary pattern, elicits detrimental effects on both metabolic and cardiovascular (CV) health. However, the role of dietary acid load (DAL) as a predictor of cardiometabolic prognosis remains to be elucidated. Thus, this study aims to compare Mediterranean diet adherence (MDA) and DAL focusing on their relationship with metabolic and CV prognosis. A total of 448 individuals aged 55–80 years were grouped depending on their MDA, assessed using food frequency questionnaires, or DAL, evaluated using potential renal load acid (PRAL) and net-endogenous acid production (NEAP). Study participants underwent anthropometric and biochemical measurements. The metabolic syndrome (MetS) prevalence was evaluated according to the National Cholesterol Education Program-Adult Treatment Panel III. Finally, the CV risk was evaluated using three independent algorithms: atherosclerotic cardiovascular disease (ASCVD), European Systematic COronary Risk Evaluation (SCORE), and Cuore risk scores. Mediterranean diet adherence was negatively associated with PRAL and NEAP. Individuals in the higher MDA tertile group had higher HDL cholesterol as well as lower homeostasis model assessment index (HOMA-IR) and fat mass relative to the lowest MDA tertile. However, in the high-MDA tertile group, there was neither a significantly lower MetS prevalence nor CV risk. Instead, both the MetS prevalence and CV risk were higher in individuals in the higher acid PRAL quartile relative to the lower alkaline PRAL quartile. Dietary acid load, especially assessed using PRAL but not MDA, was associated with indices of metabolic and CV prognosis. Thus, DAL assessed by 24-h dietary recalls may represent a better predictor of cardiometabolic health if compared to MDA evaluated using food frequency questionnaires.
Introduction
Obesity poses a huge threat to human health as well as a burden to the healthcare systems worldwide (1, 2). In fact, obesity is a key risk factor for the development of metabolic syndrome (MetS), a constellation of cardiometabolic risk factors that include central obesity, impaired glucose tolerance and insulin resistance, dyslipidemia, and hypertension (3). Unhealthy dietary patterns, alongside physical inactivity, represent the primary environmental factors to blame for the ramping up of the obesity epidemic and its cardiometabolic comorbidities. In fact, the consumption of long-chain saturated fatty acids, sugar, and processed foods, typical of the Western diet, has been widely reported to impair cardiometabolic health by fostering obesity, insulin resistance, and cardiovascular (CV) disease (4–6). On the contrary, the Mediterranean diet (MD) exerts a beneficial effect on metabolic as well as CV health (7, 8). This is because this dietary pattern is characterized by a wide consumption of plant foods (fruit, vegetables, legumes, nuts and seeds, cereals, and preferably wholegrain); the choice of seasonal, fresh, and locally grown products; the use of olive oil as the main source of added lipids; low to moderate amounts of dairy products (mainly low-fat), poultry, fish, and eggs; and a moderate intake of red wine and limited consumption of red meat and sugar (9). In concomitance with the abundance of these food groups, this dietary pattern is also abundant in a variety of food bioactives, including polyphenols, monounsaturated and polyunsaturated fatty acids, whose combinations contribute to the health-promoting effects of the MD (10).
Despite the well-documented effects of the MD on health, the adherence to this dietary pattern is declining (11) in favor of the Western diet, even in the Mediterranean area. The Western diet is a dietary pattern characterized by increased consumption of sugary drinks, red meat, highly processed foods, high-glycemic-index carbohydrates, and long-chain saturated fatty acids (12), which, as already mentioned, are metabolically detrimental. This trend is accompanied by a rise in the acid load of the diet due to an increase in protein intake, which is paralleled by a concomitant decrease in the consumption of alkalis derived from fruits and vegetables. Dietary acid load (DAL) can be computed by using different algorithms, such as potential renal acid load (PRAL) based on dietary protein, phosphorus, potassium, calcium, and magnesium intake and net-endogenous acid production (NEAP) based on protein and potassium intake.
Low and negative PRAL as opposed to high (positive) PRAL are the results of “alkaline and acidic diet,” respectively. The concept that an acidic diet is associated with negative health outcomes is rampant among the public, but scientific evidence in this context remains to be consolidated and is mainly limited to the effect of DAL on bone mass and kidney stones. In fact, the effects of an acidic diet (with high PRAL values) on pathological conditions, such as hypertension, diabetes, and CV disease, are yet to be fully elucidated (13, 14) and remain a matter of contention (15–17). While the MD has been widely demonstrated to elicit positive effects on cardiometabolic health (18–20) and may decrease DAL, it remains to identify a putative association between the MD and DAL, assessed using PRAL or NEAP. At present, there is no data in the literature that compare the impact of MDA and DAL on metabolic and CV health.
Thus, the aim of this study is to investigate the relationship between MDA or DAL and indices of metabolic and CV prognosis, such as the prevalence of MetS and CV risk in an Italian adult population.
Subjects and Methods
Participants
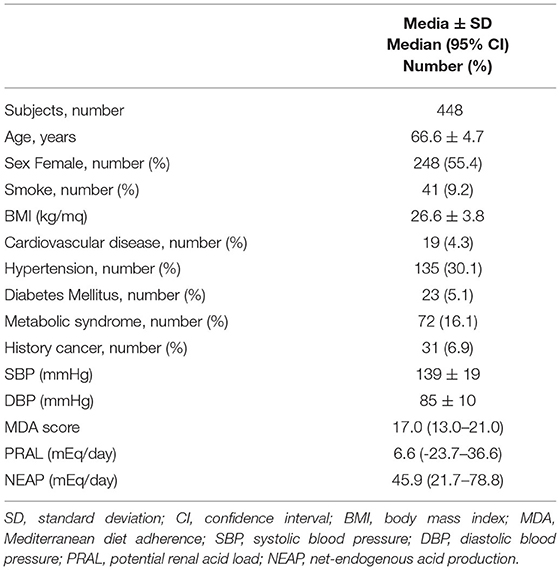
A total of 459 free-living individuals aged 55–80 years and able to walk for 2 km without any aids were enrolled in the Physical Activity and Nutrition for Quality Aging (PANGeA) study in Gemona, Trieste, and Ferrara between 2013 and 2014 (https://ec.europa.eu/regional_policy/en/projects/italy/pangea-keeping-an-aging-population-moving). Subjects having cancer, a history of hospitalization in the last 12 months, or who were taking anticoagulants were excluded. PANGEeA's participants with missing food frequency questionnaire were excluded from this study (N = 2.4%; Supplementary Figure 1). The remaining 448 subjects were clinically evaluated through interviews and physical examinations and underwent blood sampling and anthropometric measurements. The characteristics of the study population are reported in Table 1.
Written informed consent was obtained from each patient with no personal information being available to the authors (blinding). Strengthening the reporting of observational studies in epidemiology (STROBE) guidelines were followed to report observational data as well as for the preparation of this manuscript. To decrease the risk of selection bias, study participants were consecutively recruited and included in the study. Furthermore, to decrease the risk of bias, trained specific investigators were designed for the assessment of each outcome independently of the recruitment center.
This study was approved by the National Ethical Committee of the Slovenian Ministry of Health on 17 April 2012, under the acronym IR-aging 1200, and it conformed to the ethical principles for medical research involving human subjects as required by the 2013 Review of the Helsinki Declaration of Helsinki—Ethical Principles for Medical Research Involving Human Subjects.
Dietary Assessment
Assessment of Mediterranean Diet Adherence
Mediterranean diet adherence was based on a food frequency questionnaire administered to study participants by trained interviewers (nutrition expert medical doctors). The food frequency questionnaire encompassed a 90-item food and beverage list and allowed participants to indicate the consumption frequency of these items as follows: multiple times/day; 1 time/day; 5–6 times/week; 2–4 times/week; 1 time/week; 1–3 times/month, and never. To assess MDA, the consumption frequency of the following 13 main food categories was taken into consideration: milk and dairy products, cereals and grain products, vegetables, legumes, fruits, olive oil, white meat, red and processed meat, fish, sweets and desserts, nuts and seeds, and wine. Scores of zero, one, or two points indicated a low, medium, or high adherence to the MD pyramid, respectively (Supplementary Table 1). The MDA was the sum of single category scores (MDA range: 0–26 points). Scores were based on the dietary guidelines of the MD (9).
24-H Recall and DAL
Nutritional assessment was conducted through two repeated 24-h dietary recalls, which is a retrospective and quantitative method to gather information about foods and beverages consumed by the participants in 24 h prior to the visit. Two recalls were collected by trained interviewers as follows: the first one personally on the day of the visit and the second one after 2 months over the phone. Data from 24-h recall were analyzed using the nutrient analysis software Winfood® PRO 3.9.x (Medimatica Surl, Teramo, Italy) to obtain total energy and macro and micronutrients intake for each individual interview. Results were the average of the two 24-h recalls.
Data relative to nutrient intake were used to determine DAL by two algorithms, yielding PRAL (21) and NEAP (22).
Anthropometric Measurements
Anthropometric characteristics were evaluated in participants wearing light clothing with no restrictive underwear and no shoes.
Anthropometric measures included body mass index (BMI); body weight rounded to the nearest 100 g; height, waist, and hip circumferences all rounded to the nearest 0.1 cm; waist circumference measured around the smallest circumference between the lowest rib and iliac crest; hip circumference measured horizontally at the level of the greatest lateral extension of the hips.
Bioelectrical Impedance Analysis (BIA)
Body composition (total body water, fat mass, free fat mass, muscle cells, and body cell mass) and basal metabolic rate were estimated by the same trained staff member, using bioimpedance with a tetrapolar impedance meter (BIA101, Akern, Florence, Italy) according to the manufacturer's instructions (23). All measures were conducted with the patient lying down, after 8 h fasting.
Biochemical Analysis
Blood samples were collected after an overnight fast and centrifuged at 1,600 × g for 15 min at 4°C to obtain serum or plasma. Samples were aliquoted and stored at 80°C until use.
Total cholesterol, HDL cholesterol, triglycerides, glucose, and insulin were assayed using standard enzymatic-colorimetric methods (24). LDL cholesterol was calculated using Friedewald's formula (25). Insulin resistance was assessed using the homeostasis model assessment index (HOMA-IR) which was computed as follows (25):
MetS Score
Metabolic syndrome was defined according to the National Cholesterol Education Program-Adult Treatment Panel III (NCEP ATP III) and diagnosed in the presence of three or more of the following five criteria: (1) waist circumference ≥102 cm in men or ≥88 cm in women; (2) the use of antihypertensive medications, systolic blood pressure ≥130 mmHg or diastolic blood pressure ≥85 mmHg; (3) fasting triglycerides level ≥ 150 mg/dl or taking antihyperlipidaemic drugs; (4) fasting HDL cholesterol ≤ 40 mg/dl in men or ≤ 50 mg/dl in women or pharmacological treatment for low HDL cholesterol; and (5) fasting blood glucose ≥110 mg/dl or taking hypoglycaemic medications (26). MetS score ranged from 0 to 5 depending on the number of positive criteria.
Evaluation of CV Risk
The probability of having a major CV event in the 10 years post assessment was estimated for study participants without a history of CV disease (N = 429), using the following major CV risks scores: (a) atherosclerotic cardiovascular disease (ASCVD) risk score developed by the American College of Cardiology/American Heart Association Atherosclerotic Cardiovascular Disease (ACC/AHA ASCVD) and applicable to individuals aged 40–79 years (27); (b) European Systematic COronary Risk Evaluation (SCORE) developed by the European Society of Cardiology and applicable to individuals aged 45–64 years (28). The number of participants of this study with an age below 65 was 203; given the considerable impact on the participant number, the score was calculated regardless of age; and (c) Cuore risk score (Progetto CUORE individual score, National Institute of Health, Italy), based on Italian epidemiological data and applicable to individuals aged 35–69 years (29, 30).
The variables included in the calculation of those scores were age, gender, current smoking habit, TC, HDL-C (except for the European SCORE), systolic blood pressure, diagnosis of hypertension or pharmacological treatment of hypertension, and diagnosis of diabetes or taking hypoglycemic medications (except for the European SCORE). Participants with a history of CV events were excluded from risk estimation (N = 19).
Statistical Analysis
Continuous variables were analyzed for normal distribution using Shapiro–Wilk tests and expressed as mean ± standard deviation (SD) or median (95% confidence interval, CI) for normally and non-normally distributed variables, respectively. One-way ANOVA or Kruskal–Wallis tests were used to assess overall differences between groups, and Dunnet or Mann–Whitney tests were performed for comparisons between extreme tertiles or quartiles (high MDA vs. low MDA and strong PRAL vs. alkaline PRAL). Categorical variables were compared with exact Fisher or chi-squared tests. Spearman's correlation coefficient was used to test the association between MDA, PRAL, or NEAP and the parameters of interest. A p-value of ≤ 0.05 was considered statistically significant.
Missing data for each variable of interest did not exceed 5%.
Results
Study Population and Dietary Assessment
Assessment of MDA revealed that only 20 individuals included in the study (4.5%) had a score lower than 13 points (the arithmetic means of the 0–26 MDA range), while half of the study participants had an MDA score ranging between 15 and 18 points (Table 1). The scores of the 13 main food categories used to calculate the MDA score are reported in Supplementary Table 1. Compliance with the guidelines set out in the MD pyramid was higher for the consumption of vegetables, milk and dairy products, wheat, and fruit (Supplementary Table 2). Instead, the lowest compliance was observed relative to the consumption of nuts and sweets. Another poorly followed MD recommendation concerned the consumption of processed and red meat. The minimum score (zero) for the consumption of these foods was obtained by 176 participants (39.3%), of which 6 (1.3%) almost never ate red or processed meat, while the other 169 (37.7%) ate it more than 4 times a week.
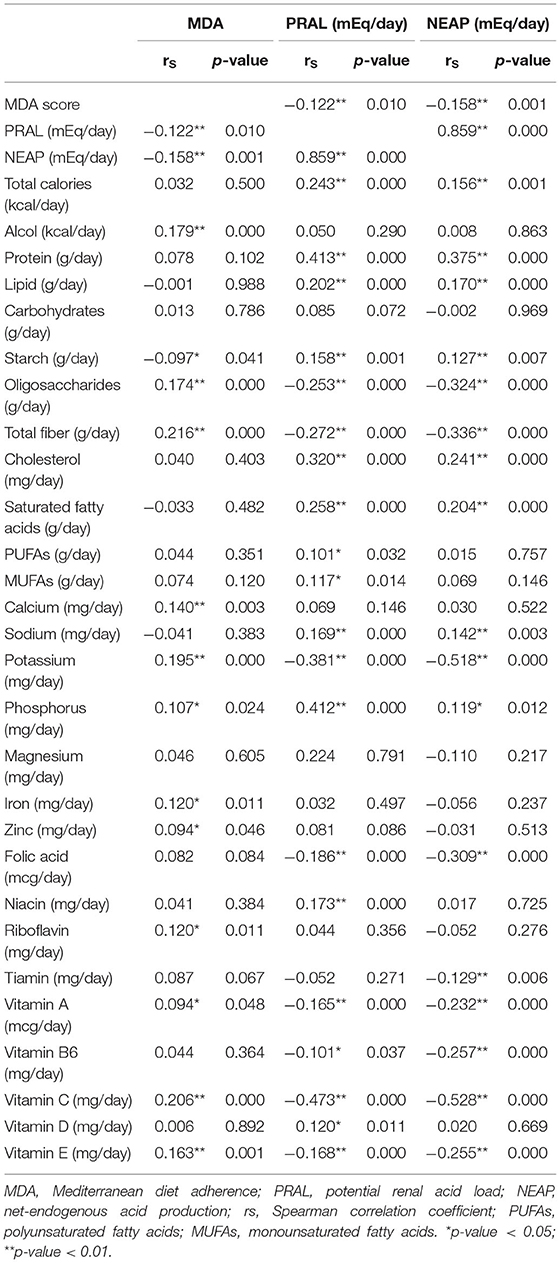
Correlation analysis between MDA score, PRAL, and NEAP as well as energy, micronutrient, and macronutrient intake is depicted in Table 2. MDA correlated negatively with PRAL and NEAP. There was no association between the total energy intake and MDA. Instead, greater adherence to MD was positively associated with the intake of total dietary fiber; oligosaccharides; microelements such as calcium, potassium, phosphorous, iron, and zinc; and vitamins such as riboflavin, thiamine, vitamin C, and vitamin E. Furthermore, MDA was correlated positively with alcohol intake, which is in agreement with the fact that the mild and regular consumption of wine at mealtimes is one of the cornerstones of the MD.
As expected, there was a strong positive and statistically significant correlation between PRAL and NEAP. These DAL-related parameters showed a positive association with the intake of total calories, protein, starch, lipid (total cholesterol and saturated lipid), sodium, and phosphorous. On the contrary, an increase in DAL was associated with a decrease in the intake of oligosaccharides, total dietary fiber, potassium, and some vitamins (folic acid, thiamine, vitamin B6, vitamin C, vitamin A, and vitamin E). Despite both PRAL and NEAP being related to DAL, there were differences between these DAL proxies. Only PRAL was positively correlated with monounsaturated and polyunsaturated fatty acids, niacin, and vitamin D intake, while only NEAP was negatively correlated with tiamin (Table 2).
Anthropometrics, Body Composition, and Metabolic Parameters
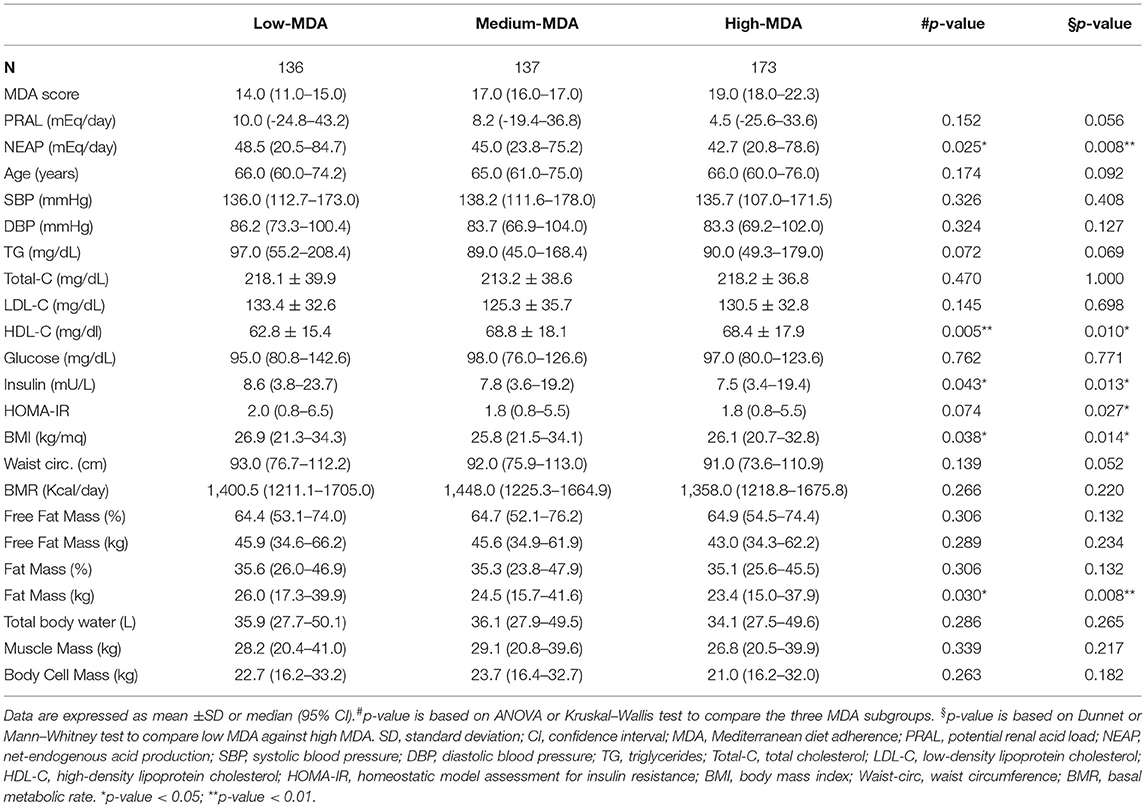
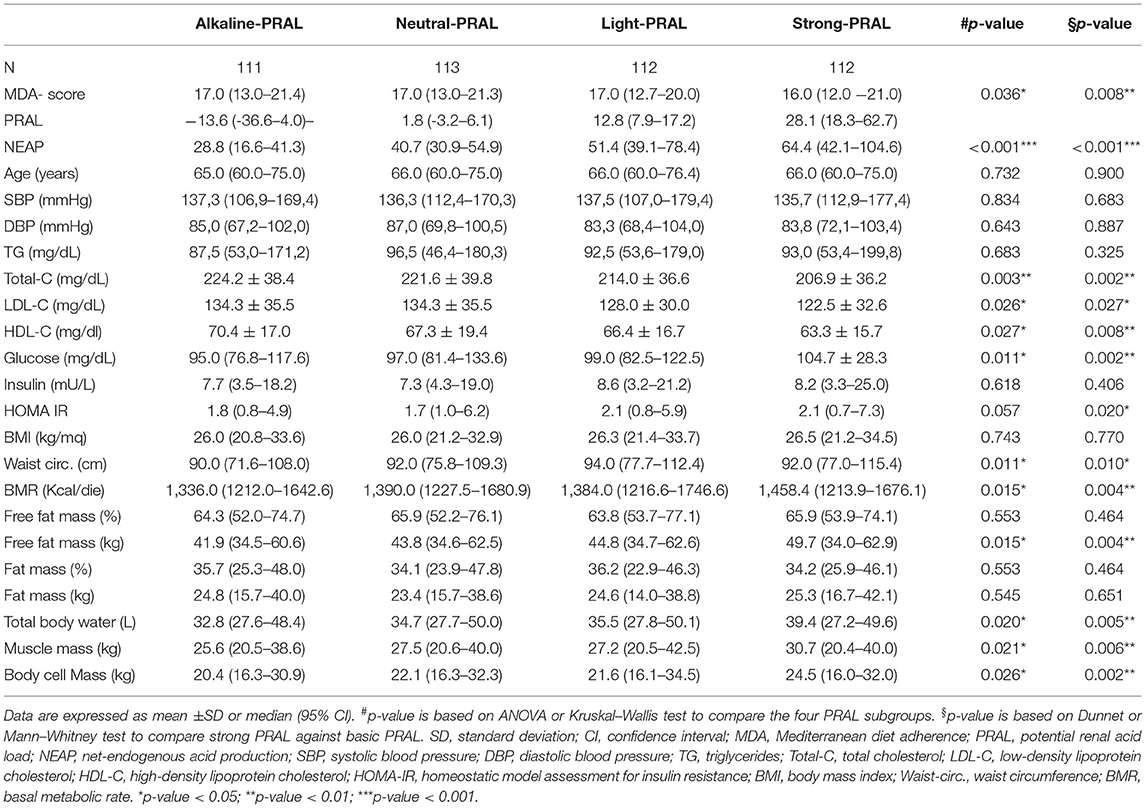
To investigate the effects of dietary habits on selected health outcomes, PRAL was divided into quartiles to associate this parameter with an alkaline, neutral, slightly acidic, or strongly acidic diet (alkaline PRAL, neutral PRAL, light PRAL, and strong PRAL, respectively). Similar to PRAL, NEAP was divided into quartiles. Instead, the MDA score range of the study participants was too tight to separate them into quartiles; therefore, they were divided into tertiles as follows: low MDA, medium MDA, and high MDA.
Participants in the low-MDA tertile, relative to individuals in the high-MDA tertiles, had a higher BMI and fat mass; a worse metabolic profile marked by higher triglyceride concentrations, lower HDL cholesterol, and higher HOMA-IR values. Furthermore, study participants in the low-MDA tertile had a higher DAL, as assessed by NEAP, compared to participants in the high-MDA tertile (Table 3). Despite the rest of the parameters analyzed did not reach statistical significance between MDA tertiles, the differences in waist circumference, triglycerides, and PRAL tended to be significant when comparing individuals in the low- and high-MDA tertiles (p = 0.052, p = 0.069, and p = 0.056, respectively).
When DAL was used to compare the metabolic and anthropometric characteristics of the study participants, individuals in the highest PRAL quartile (strong PRAL) (Table 4) were characterized by lower total, LDL, and HDL cholesterol concentrations; higher waist circumference; free fat mass; glucose concentration; and HOMA-IR (Table 4). Anthropometric data analysis revealed higher basal metabolic rate, muscle cell, and body cell mass in strong PRAL vs. alkaline PRAL.
MetS Score
Mediterranean diet adherence tertiles and PRAL quartiles were also used to evaluate the impact of the MDA and DAL on MetS prevalence and MetS score (Figure 1, Supplementary Tables 3A,B). MetS prevalence was not significantly affected by MDA, with a similar trend being observed for the MetS score. Instead, individuals in the strong PRAL quartile had a higher prevalence of the MetS, which is explained by an increase in the number of participants with positive criteria for MetS diagnosis, including blood pressure, triglycerides, and fasting glucose. Similar results were obtained by stratifying the study participants according to NEAP values.
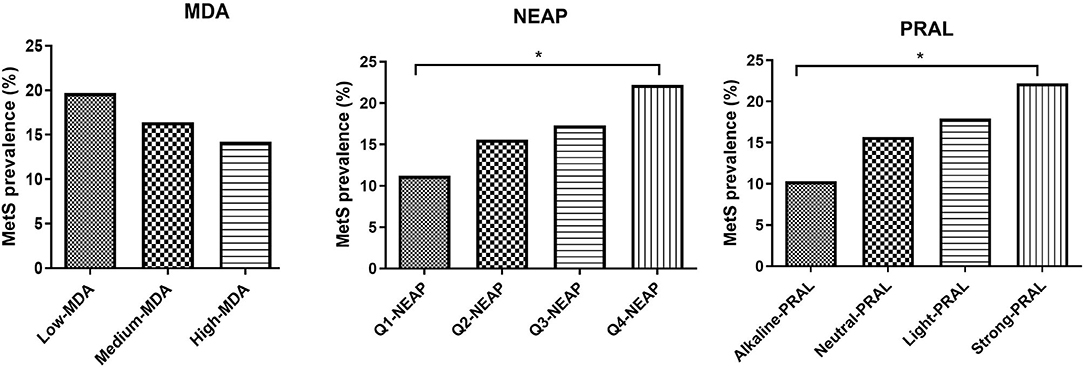
Figure 1. MetS prevalence divided by MDA tertiles NEAP quartiles and PRAL quartiles. Data are expressed as percentage of metabolic syndrome prevalence and analyzed using Fisher's exact test. *p-value < 0.05. MDA, Mediterranean diet adherence; PRAL, potential renal acid load; NEAP, net-endogenous acid production; MetS, metabolic syndrome.
CV Risk Score
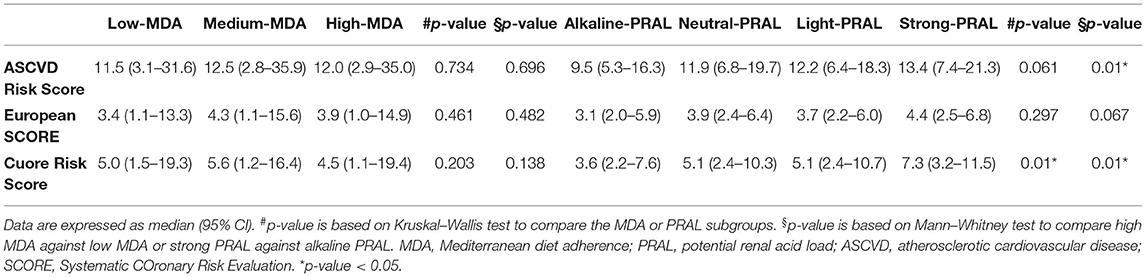
The effect of eating habits on CV risk was evaluated by considering 3 different calculation risk tools (Table 5, Supplementary Table 4). As described for MetS, MDA did not affect the CV risk score independently of the algorithm used. On the contrary, the strong PRAL group had significantly higher ASCVD and CUORE scores relative to alkaline PRAL. The European SCORE (ESC) risk score was also higher in participants in the strong PRAL quartile, but it did not reach significance (p = 0.067). Higher NEAP was associated with higher ASCVD and CUORE scores (Supplementary Table 4).
Discussion
This study aimed at shedding light on the relationship between MetS, estimated CV risk, and dietary habits, with a particular focus on MDA and DAL.
The results reported in this study indicate that the MDA score negatively correlates with DAL. Nonetheless, despite MDA being associated with an improvement in some metabolic health parameters, a higher MDA score was not concomitant with a lower prevalence of MetS or a decrease in CV risk, an effect which was observed in the study participants consuming diets with a low DAL. The statistical analyses have generally shown similar results for PRAL and NEAP, but with a higher statistical power for PRAL.
At present, to our knowledge, no study has analyzed the relationship between MDA score, DAL, and cardiometabolic health, especially to test the possibility that some of the metabolic benefits of the MD may be dependent on its low DAL. However, Bullò and coworkers (31) partially addressed this matter by investigating the effects of a 1-year intervention with an MD supplemented with olive oil or nuts on bone health. The authors reported a decrease in PRAL and NEAP values in the participants eating an MD supplemented with olive oil and an increase in those consuming an MD integrated with nuts (31). This is in line with the findings of this study, in which a higher MDA score was associated with lower PRAL and NEAP and the fact that the study participants reported high olive oil and low nut consumption.
The health-promoting effects ascribed to the MD appear to be mediated, at least in part, by the high intake of antioxidants derived from fruits, olive oil, and vegetables, as well as the intake of oleic acid, dietary fiber, non-refined carbohydrates, and plant proteins (32). These key nutritional features are reflected in this study, as indicated by a positive association between MDA score and the intake of vitamins with antioxidant activity (vitamin A, vitamin C, and vitamin E), dietary fiber, and potassium. Instead, these nutrients negatively correlated with PRAL. Furthermore, while no association was observed relative to the MDA score, NEAP and PRAL were positively correlated with saturated fatty acids and cholesterol intake. In light of this, while antioxidants and dietary fibers may contribute to the cardiometabolic protection exerted by the MD (33, 34), saturated fatty acids and cholesterol represent key elements in the development of cardiometabolic diseases (35). However, the MDA score did not correlate with saturated (negatively) and monounsaturated (positively) fatty acids. This may be explained by the fact that none of the study participants completely adhered to the MD, as witnessed by MDA scores ranging between 18.0 and 22.3 out of 26.
As already described by the EPIC-PANACEA project (36), the results presented in this study confirm the positive impact of the MD on body weight regulation. Study participants with a high-MDA score had a lower BMI and fat mass compared to participants in the low-MDA tertile. Instead, there were no differences in BMI and fat mass between participants stratified by PRAL values, although there were significant differences in waist circumference, free fat mass, total body water, muscle mass, and body cell mass between these groups. Findings described in the literature about the associations between DAL and BMI are controversial. An umbrella review by Farhangi et al. (37) reported either a positive, negative or no association between DAL and BMI. After analyzing all the data, they concluded that significant differences were observed only when participants were stratified by sex; higher BMI was associated with higher PRAL in women and with higher NEAP in men (37). Nevertheless, BMI is not a sufficient predictor of cardiometabolic outcomes as it does not take into consideration body composition nor fat distribution. In fact, differences in BMI may also reflect variations in fat-free mass and muscle mass, which increase in parallel with DAL and may be dependent on the higher protein intake. On the contrary, waist circumference represents a more accurate predictor of cardiometabolic health compared to BMI alone (38). Not surprisingly, in fact, study participants in the highest PRAL quartile had higher waist circumference measures, which supports the potential association between a higher DAL and cardiometabolic diseases.
In agreement with a previous meta-analysis (39), the results reported in this study revealed a better metabolic profile in participants with a higher MDA score. This was underlined by a lower HOMA-IR, suggesting an increase in insulin sensitivity and an improvement in the circulating levels of HDL cholesterol and triglycerides, albeit the latter did not reach statistical significance. Despite this, a higher MDA score was not associated with a reduction in the criteria for the diagnosis of MetS or MetS prevalence. On the contrary, the percentage of study participants with positive MetS diagnostic criteria, namely, circulating glucose, triglycerides levels, and blood pressure were higher in the strong PRAL group vs. the alkaline PRAL group, supporting the possibility that a higher DAL may impair metabolic health (16). Similar results were obtained by analyzing NEAP quartiles and MetS prevalence. These results are in agreement with previous reports indicating similar associations between PRAL as well as NEAP and the prevalence of MetS in Iranian patients with type 2 diabetes (40) and a cross-sectional Japanese study, in which higher NEAP values (PRAL was not calculated) were associated with an increased prevalence of MetS independently of sex, age, and BMI (17). Contradictory results were described by Jafari et al. (41) who observed an association between MetS prevalence and NEAP but not PRAL in a cohort of Iranian men. Instead, the other two Iranian studies reported no association between PRAL or NEAP and the MetS (42, 43). Diverging results may be due to differences in dietary quality and/or genetic, sociodemographic, and behavioral characteristics of the population. Furthermore, the lack of association between MDA and decrease in MetS prevalence may be dependent on the fact that the diet of the study cohort, including for the individuals with a higher MDA score, was not always strictly in line with the MD pyramid, as already described.
In this study, higher PRAL and NEAP values were associated with a heightened CV risk regardless of the risk score used. Similar results were reported in the only study conducted to date (44). Regarding the MDA score, instead, no association with CV risk was observed. However, this is surprising, especially considering this dietary pattern being widely demonstrated to exert protective effects against major CV events (7). The reason for this discrepancy may be dependent on the fact that this study was not a strict dietary intervention, but it is based on food frequency questionnaires and by the fact that the recommendations set by the MD pyramid were not always followed by a large portion of the study population, as in the case of red meat consumption. Furthermore, while a Mediterranean diet supplemented with nuts has previously been shown to increase DAL compared to an MD supplemented with olive oil (31), it still exerted a protective effect against CV events (7). This suggests that despite DAL being associated with CV risk, alone it is not sufficient to explain CV risk. Instead, CV risk is more likely to depend upon overall diet quality. In fact, in this study, a higher DAL was positively associated with the intake of total lipids, saturated fatty acids, cholesterol, and sodium, with all these nutrients being linked with an increase in CV risk (45). Thus, in this study, a low DAL may represent an indicator of healthier dietary choices linked with better cardiometabolic health. This is in agreement with the metabolically detrimental effects of the Western diet, which, in fact, is a highly acidogenic dietary pattern (16, 46).
The results described here allow various reflections. First, it is possible to state that adherence to the MD, measured as the frequency of food consumption without considering the number of nutrients ingested, does not reflect MetS prevalence nor CV risk. An increase in the predictive power could be given by considering not only the diet but also other parameters associated with the Mediterranean lifestyle, such as culinary, social, and physical activity habits. An example of this is the MEDLIFE index, developed by Sotos-Prieto et al. (47) which includes fifteen items relative to food consumption; seven items about traditional Mediterranean dietary habits; and six items about physical activity, rest, and social interactions.
Second, especially when assessed by PRAL, DAL was associated with worse indices of metabolic and CV prognosis. Positive linear associations of PRAL and pathological conditions are described in literature except in a studio by Xu et al. (48) in which a modest non-linear U-shaped relation between mortality rates and PRAL was found, with a worse prognosis for both dietary acid and alkali excess. However, in this study, alkaline PRAL values are lower than values observed in this study (minimal values of PRAL were −111 and −41.9 mEq/day, respectively). Therefore, the present results could not exclude potential negative effects of alkali excess in the diet.
This study has several strengths. First, the MDA score, PRAL, and NEAP were estimated based on questionnaires and algorithms administered or calculated by trained interviewers and not self-administered questionnaires as reported in other studies. Second, DAL was assessed by two tools (PRAL and NEAP), while the CV risk score was calculated by 3 different validated algorithms, and the results were similar. Third, participants comprised a relatively large and well-characterized population. Fourth, to the best of our knowledge, this was the first study to investigate the relationship between indices of metabolic and CV health and dietary parameters, namely MDA score and DAL.
This study also presents some limitations, which need to be taken into consideration. First, the lack of consensus on how to assess MDA made it difficult to compare the present results with previously published studies reporting on MDA scores. In fact, there are almost 30 scores based on different food frequency questionnaires to assess MDA (49). PRAL and NEAP are calculated by a mathematical equation using levels of nutrients estimated with software (Winfood) using the average of the two 24-h recalls.
Conclusion
There is no doubt that the MD pattern is associated with lower CV risk, but the MDA score calculated using food frequency did not correlate with metabolic and CV state defined as the prevalence of MetS and estimated CV risk. Instead, DAL values, especially those assessed using PRAL computed from 24-h recalls, more closely relate to a cardiometabolic health state.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors upon reasonable request.
Ethics Statement
The study involving human participants was approved by National Ethical Committee of the Slovenian Ministry of Health and reviewed and approved by Comitato Etico di Area Vasta Emilia Centro (CE-AVEC). The participants provided their written informed consent to participate in this study.
Author Contributions
JS and AP: design of the study and data analysis. JS, SC, EC, RS, and FGDG: acquisition of data. JS, DS, and AP: data interpretation. JS and DS: drafting of the article. GB, SL, BS, RP, and AP: critical revision of the article. All authors read and approved the final manuscript.
Funding
This study was a part of the research project Physical Activity and Nutrition for Quality Aging (PANGeA), supported by a grant from the Cross-border Cooperation Program Slovenia, Italy 2007–2013, grant number 042-2/2009.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank the participants in the study for their time and effort to ensure the success of the project. We acknowledge the excellent assistance of the research team of the PANGeA mass measurement (Italy—University of Ferrara: Edoardo dalla Nora, Gloria Brombo, Cecilia Soavi, Elettra Mantovani, Mario Luca Morieri, Maria Agata Miselli, and Daniela Francesconi; University of Udine: Giovanelli Nicola, Mirco Floreani, Martina Arteni, Alberto Botter, and Desy Salvadego; University of Trieste: Mariella Sturma, Giuseppe Castiglia, Marcello Tence, Greta del Fabbro, Sara Mazzucco, and Paolo de Colle; Slovena—Science and Research Centre of Koper: Uroš Marušič, Matej Plevnik, Saša Pišot, Dorjana Zerbo, Nina Mohorko, and Petra Dolenc; General Hospital of Isola: Mladen Gasparini; National Institute for Public Health; Mojca Gabrijelčič Blenkuš). Additionally, we thank the entire staff for their help and logistic support and many other researchers and colleagues from different institutes and different countries who contributed to the smooth undertaking of the study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.828587/full#supplementary-material
References
1. Brown WV, Fujioka K, Wilson PW, Woodworth KA. Obesity: why be concerned? Am J Med. (2009) 122:S4–11. doi: 10.1016/j.amjmed.2009.01.002
2. Friedrich MJ. Global obesity epidemic worsening. JAMA. (2017) 318:603. doi: 10.1001/jama.2017.10693
3. Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. (2005) 365:1415–28. doi: 10.1016/S0140-6736(05)66378-7
4. Tindall AM, Petersen KS, Skulas-Ray AC, Richter CK, Proctor DN, Kris-Etherton PM. Replacing saturated fat with walnuts or vegetable oils improves central blood pressure and serum lipids in adults at risk for cardiovascular disease: a randomized controlled-feeding trial. J Am Heart Assoc. (2019) 8:e011512. doi: 10.1161/JAHA.118.011512
5. Sergi D, Williams LM. Potential relationship between dietary long-chain saturated fatty acids and hypothalamic dysfunction in obesity. Nutr Rev. (2020) 78:261–77. doi: 10.1093/nutrit/nuz056
6. Machado PP, Steele EM, Levy RB, da Costa Louzada ML, Rangan A, Woods J, et al. Ultra-processed food consumption and obesity in the Australian adult population. Nutr Diabetes. (2020) 10:39. doi: 10.1038/s41387-020-00141-0
7. Estruch R, Ros E, Salas-Salvado J, Covas MI, Corella D, Aros F, et al. Primary prevention of cardiovascular disease with a mediterranean diet. N Engl J Med. (2013) 368:1279–90. doi: 10.1056/NEJMoa1200303
8. Lotfi K, Saneei P, Hajhashemy Z, Esmaillzadeh A. Adherence to the mediterranean diet, five-year weight change, and risk of overweight and obesity: a systematic review and dose-response meta-analysis of prospective cohort studies. Adv Nutr. (2021) 13:152–66. doi: 10.1093/advances/nmab092
9. Bach-Faig A, Berry EM, Lairon D, Reguant J, Trichopoulou A, Dernini S, et al. Mediterranean diet pyramid today. science and cultural updates. Public Health Nutr. (2011) 14:2274–84. doi: 10.1017/S1368980011002515
10. Saura-Calixto F, Goni I. Definition of the mediterranean diet based on bioactive compounds. Crit Rev Food Sci Nutr. (2009) 49:145–52. doi: 10.1080/10408390701764732
11. Vilarnau C, Stracker DM, Funtikov A, da Silva R, Estruch R, Bach-Faig A. Worldwide adherence to mediterranean diet between 1960 and 2011. Eur J Clin Nutr. (2019) 72:83–91. doi: 10.1038/s41430-018-0313-9
12. Cordain L, Eaton SB, Sebastian A, Mann N, Lindeberg S, Watkins BA, et al. Origins and evolution of the western diet: health implications for the 21st century. Am J Clin Nutr. (2005) 81:341–54. doi: 10.1093/ajcn.81.2.341
13. Parohan M, Sadeghi A, Nasiri M, Maleki V, Khodadost M, Pirouzi A, et al. Dietary acid load and risk of hypertension: a systematic review and dose-response meta-analysis of observational studies. Nutr Metab Cardiovasc Dis. (2019) 29:665–75. doi: 10.1016/j.numecd.2019.03.009
14. Dehghan P, Abbasalizad Farhangi M. Dietary acid load, blood pressure, fasting blood sugar and biomarkers of insulin resistance among adults: findings from an updated systematic review and meta-analysis. Int J Clin Pract. (2020) 74:e13471. doi: 10.1111/ijcp.13471
15. Xu H, Jia T, Huang X, Riserus U, Cederholm T, Arnlov J, et al. Dietary acid load, insulin sensitivity and risk of type 2 diabetes in community-dwelling older men. Diabetologia. (2014) 57:1561–8. doi: 10.1007/s00125-014-3275-z
16. Williams RS, Kozan P, Samocha-Bonet D. The role of dietary acid load and mild metabolic acidosis in insulin resistance in humans. Biochimie. (2016) 124:171–7. doi: 10.1016/j.biochi.2015.09.012
17. Arisawa K, Katsuura-Kamano S, Uemura H, Tien NV, Hishida A, Tamura T, et al. Association of dietary acid load with the prevalence of metabolic syndrome among participants in baseline survey of the japan multi-institutional collaborative cohort study. Nutrients. (2020) 12:1605. doi: 10.3390/nu12061605
18. Martin-Pelaez S, Fito M, Castaner O. Mediterranean diet effects on type 2 diabetes prevention, disease progression, and related mechanisms. a review. Nutrients. (2020) 12:2236. doi: 10.3390/nu12082236
19. Hershey MS, Sotos-Prieto M, Ruiz-Canela M, Christophi CA, Moffatt S, Martinez-Gonzalez MA, et al. The mediterranean lifestyle (MEDLIFE) index and metabolic syndrome in a non-mediterranean working population. Clin Nutr. (2021) 40:2494–503. doi: 10.1016/j.clnu.2021.03.026
20. Martinez-Gonzalez MA, Gea A, Ruiz-Canela M. The mediterranean diet and cardiovascular health. Circ Res. (2019) 124:779–98. doi: 10.1161/CIRCRESAHA.118.313348
21. Remer T, Dimitriou T, Manz F. Dietary potential renal acid load and renal net acid excretion in healthy, free-living children and adolescents. Am J Clin Nutr. (2003) 77:1255–60. doi: 10.1093/ajcn/77.5.1255
22. Frassetto LA, Todd KM, Morris RC, Sebastian A. Estimation of net endogenous noncarbonic acid production in humans from diet potassium and protein contents. Am J Clin Nutr. (1998) 68:576–83. doi: 10.1093/ajcn/68.3.576
23. Lukaski HC, Johnson PE, Bolonchuk WW, Lykken GI. Assessment of fat-free mass using bioelectrical impedance measurements of the human body. Am J Clin Nutr. (1985) 41:810–7. doi: 10.1093/ajcn/41.4.810
24. Passaro A, Soavi C, Marusic U, Rejc E, Sanz JM, Morieri ML, et al. Computerized cognitive training and brain derived neurotrophic factor during bed rest: mechanisms to protect individual during acute stress. Aging. (2017) 9:393–407. doi: 10.18632/aging.101166
25. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. (1972) 18:499–502. doi: 10.1093/clinchem/18.6.499
26. Grundy SM, Brewer HB, Cleeman JI, Smith SC, Lenfant C, American Heart Association, et al. Definition of metabolic syndrome: report of the national heart, lung, and blood institute/American heart association conference on scientific issues related to definition. Circulation. (2004) 109:433–8. doi: 10.1161/01.CIR.0000111245.75752.C6
27. Goff DC, Lloyd-Jones DM, Bennett G, Coady S, D'Agostino RB, Gibbons R, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American college of cardiology/American heart association task force on practice guidelines. Circulation. (2014) 129:S49–73. doi: 10.1161/01.cir.0000437741.48606.98
28. Conroy RM, Pyorala K, Fitzgerald AP, Sans S, Menotti A, De Backer G, et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. (2003) 24:987–1003. doi: 10.1016/S0195-668X(03)00114-3
29. Donfrancesco C, Palmieri L, Cooney MT, Vanuzzo D, Panico S, Cesana G, et al. Italian cardiovascular mortality charts of the CUORE project: are they comparable with the SCORE charts? Eur J Cardiovasc Prev Rehabil. (2010) 17:403–9. doi: 10.1097/HJR.0b013e328334ea70
30. Palmieri L, Panico S, Vanuzzo D, Ferrario M, Pilotto L. La valutazione del rischio cardiovascolare globale assoluto: il punteggio individuale del Progetto CUORE. Annali dell'Istituto superiore di sanità. (2004) 40:393–9.
31. Bullo M, Amigo-Correig P, Marquez-Sandoval F, Babio N, Martinez-Gonzalez MA, Estruch R, et al. Mediterranean diet and high dietary acid load associated with mixed nuts: effect on bone metabolism in elderly subjects. J Am Geriatr Soc. (2009) 57:1789–98. doi: 10.1111/j.1532-5415.2009.02481.x
32. Chatzianagnostou K, Del Turco S, Pingitore A, Sabatino L, Vassalle C. The mediterranean lifestyle as a non-pharmacological and natural antioxidant for healthy aging. Antioxidants. (2015) 4:719–36. doi: 10.3390/antiox4040719
33. Vatner SF, Zhang J, Oydanich M, Berkman T, Naftalovich R, Vatner DE. Healthful aging mediated by inhibition of oxidative stress. Ageing Res Rev. (2020) 64:101194. doi: 10.1016/j.arr.2020.101194
34. Buil-Cosiales P, Toledo E, Salas-Salvado J, Zazpe I, Farras M, Basterra-Gortari FJ, et al. Association between dietary fibre intake and fruit, vegetable or whole-grain consumption and the risk of CVD: results from the PREvencion con DIeta MEDiterranea (PREDIMED) trial. Br J Nutr. (2016) 116:534–46. doi: 10.1017/S0007114516002099
35. Zock PL, Blom WA, Nettleton JA, Hornstra G. Progressing insights into the role of dietary fats in the prevention of cardiovascular disease. Curr Cardiol Rep. (2016) 18:111. doi: 10.1007/s11886-016-0793-y
36. Romaguera D, Norat T, Vergnaud AC, Mouw T, May AM, Agudo A, et al. Mediterranean dietary patterns and prospective weight change in participants of the EPIC-PANACEA project. Am J Clin Nutr. (2010) 92:912–21. doi: 10.3945/ajcn.2010.29482
37. Abbasalizad Farhangi M, Nikniaz L, Nikniaz Z. Higher dietary acid load potentially increases serum triglyceride and obesity prevalence in adults: an updated systematic review and meta-analysis. PLoS ONE. (2019) 14:e0216547. doi: 10.1371/journal.pone.0216547
38. Klein S, Allison DB, Heymsfield SB, Kelley DE, Leibel RL, Nonas C, et al. Waist circumference and cardiometabolic risk: a consensus statement from shaping America's health: association for weight management and obesity prevention; NAASO, the obesity society; the American society for nutrition; and the American diabetes association. Diabetes Care. (2007) 30:1647–52. doi: 10.2337/dc07-9921
39. Bakaloudi DR, Chrysoula L, Kotzakioulafi E, Theodoridis X, Chourdakis M. Impact of the level of adherence to mediterranean diet on the parameters of metabolic syndrome: a systematic review and meta-analysis of observational studies. Nutrients. (2021) 13:1514. doi: 10.3390/nu13051514
40. Iwase H, Tanaka M, Kobayashi Y, Wada S, Kuwahata M, Kido Y, et al. Lower vegetable protein intake and higher dietary acid load associated with lower carbohydrate intake are risk factors for metabolic syndrome in patients with type 2 diabetes: post-hoc analysis of a cross-sectional study. J Diabetes Investig. (2015) 6:465–72. doi: 10.1111/jdi.12326
41. Jafari A, Ghanbari M, Shahinfar H, Bellissimo N, Azadbakht L. The association between dietary acid load with cardiometabolic risk factors and inflammatory markers amongst elderly men: a cross-sectional study. Int J Clin Pract. (2021) 75:e14109. doi: 10.1111/ijcp.14109
42. Mozaffari H, Namazi N, Larijani B, Bellissimo N, Azadbakht L. Association of dietary acid load with cardiovascular risk factors and the prevalence of metabolic syndrome in Iranian women: a cross-sectional study. Nutrition. (2019) 67–8:110570. doi: 10.1016/j.nut.2019.110570
43. Mohammadifard N, Karimi G, Khosravi A, Sarrafzadegan N, Jozan M, Zahed P, et al. High dietary acid load score is not associated with the risk of metabolic syndrome in Iranian adults. Int J Vitam Nutr Res. (2021) 91:152–63. doi: 10.1024/0300-9831/a000626
44. Han E, Kim G, Hong N, Lee YH, Kim DW, Shin HJ, et al. Association between dietary acid load and the risk of cardiovascular disease: nationwide surveys (KNHANES 2008-2011). Cardiovasc Diabetol. (2016) 15:122. doi: 10.1186/s12933-016-0436-z
45. Calabrese I, Riccardi G. Effectiveness of changes in diet composition on reducing the incidence of cardiovascular disease. Curr Cardiol Rep. (2019) 21:88. doi: 10.1007/s11886-019-1176-y
46. Drake I, Sonestedt E, Ericson U, Wallstrom P, Orho-Melander M. A western dietary pattern is prospectively associated with cardio-metabolic traits and incidence of the metabolic syndrome. Br J Nutr. (2018) 119:1168–76. doi: 10.1017/S000711451800079X
47. Sotos-Prieto M, Moreno-Franco B, Ordovas JM, Leon M, Casasnovas JA, Penalvo JL. Design and development of an instrument to measure overall lifestyle habits for epidemiological research: the mediterranean lifestyle (MEDLIFE) index. Public Health Nutr. (2015) 18:959–67. doi: 10.1017/S1368980014001360
48. Xu H, Akesson A, Orsini N, Hakansson N, Wolk A, Carrero JJ. Modest U-Shaped association between dietary acid load and risk of all-cause and cardiovascular mortality in adults. J Nutr. (2016) 146:1580–5. doi: 10.3945/jn.116.231019
Keywords: Mediterranean diet, dietary acid load, PRAL, NEAP, alkaline diet, acidic diet, metabolic syndrome, cardiovascular risk score
Citation: Sanz JM, Sergi D, Colombari S, Capatti E, Situlin R, Biolo G, Di Girolamo FG, Lazzer S, Šimunič B, Pišot R and Passaro A (2022) Dietary Acid Load but Not Mediterranean Diet Adherence Score Is Associated With Metabolic and Cardiovascular Health State: A Population Observational Study From Northern Italy. Front. Nutr. 9:828587. doi: 10.3389/fnut.2022.828587
Received: 03 December 2021; Accepted: 14 March 2022;
Published: 26 April 2022.
Edited by:
Kim Bell-Anderson, The University of Sydney, AustraliaReviewed by:
Arrigo Francesco Giuseppe Cicero, University of Bologna, ItalyD'Addato Sergio, University of Bologna, Italy
Copyright © 2022 Sanz, Sergi, Colombari, Capatti, Situlin, Biolo, Di Girolamo, Lazzer, Šimunič, Pišot and Passaro. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Angelina Passaro, angelina.passaro@unife.it
 Juana Maria Sanz
Juana Maria Sanz Domenico Sergi
Domenico Sergi Simona Colombari3
Simona Colombari3  Gianni Biolo
Gianni Biolo Filippo Giorgio Di Girolamo
Filippo Giorgio Di Girolamo Stefano Lazzer
Stefano Lazzer Boštjan Šimunič
Boštjan Šimunič